Intellectual Property: Information on Draft Guidance to Assert Government Rights Based on Price
Fast Facts
When federally funded research leads to inventions, there are certain conditions under which the funding agency can exercise "march-in rights." March-in rights permit a funding agency to allow a third party to license the invention.
In 2023, the National Institute of Standards and Technology released draft guidance proposing that the government could consider the price of a product when deciding whether to use march-in rights. Most public comments on the guidance supported using march-in rights to lower the cost of prescription drugs. Studies estimate this would only affect a few drugs.
As of December 2025, NIST hadn't finalized the guidance.
NIST's campus in Gaithersburg, Maryland

A sign that says: National Institute of Standards and Technology, U.S. Department of Commerce
Highlights
What GAO Found
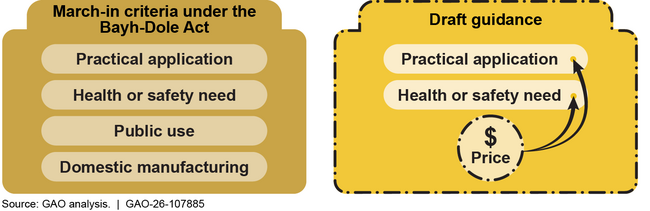
Under the Bayh-Dole Act of 1980, federal agencies can, in certain circumstances, exercise the authority known as march-in rights when an invention that arose from federally funded research is involved. March-in-rights entail an agency requiring a recipient of its funding to issue a license to a third party to develop the invention. Agencies have never exercised march-in rights. In December 2023, the National Institute of Standards and Technology (NIST) published draft guidance that sought to clarify when agencies could exercise this authority. It proposed using the price of a product resulting from a federally funded invention as a factor for exercising march-in rights. According to the guidance, price could be used under two of the four statutory criteria: practical application and health or safety need (see figure).
The draft guidance was developed through a NIST-led interagency process. As of December 2025, NIST did not have a timeline for finalizing the guidance, citing a lack of interagency consensus.
NIST Draft Guidance Proposed Using Price as a Factor Under Two Statutory Criteria for Exercising March-In Rights
Among the 51,762 public comments on the draft guidance, more than 47,000 comments (about 91 percent) expressed support for the draft guidance, with the remainder expressing opposition. Most comments in favor of the guidance expressed concern about high prescription drug prices and support for using march-in rights to lower them. Comments opposing the guidance—including all comments submitted by universities—raised concerns about potential adverse effects, such as reducing universities’ ability to license inventions and businesses’ ability to attract investment to develop the inventions into products.
Because march-in rights have never been exercised, it is only possible to discuss hypothetical impacts of implementing the draft guidance. A federal agency could exercise march-in rights based on product price only if a product resulting from a federally funded invention has an unexpired patent subject to Bayh-Dole. Therefore, the potential for march-in is higher for technologies with a high volume of patenting activity arising from federally funded research, such as pharmaceuticals, computer technology, and electrical machinery. Although most public comments on the draft guidance expressed support for using march-in rights to lower drug prices, studies estimate that march-in based on price would likely affect a small number of drugs. This is because most drugs have patents that are not subject to Bayh-Dole.
Why GAO Did This Study
Federal agencies fund universities and other organizations to conduct research, which can lead to new inventions. Under the Bayh-Dole Act, recipients of federal funding can retain patent rights to the inventions and license them to other parties. To protect public interest in these inventions, the act allows federal agencies to retain certain rights, including march-in rights. These permit an agency to require a recipient to issue a license to a third party, when the circumstances meet at least one of four criteria specified in the act. If the recipient refuses, the agency itself can grant a license.
Agencies can initiate march-in proceedings on their own or in response to requests from external parties. Since the passage of the Bayh-Dole Act, agencies have received about a dozen march-in requests; most of these addressed lowering the price of drugs or other medical technologies. For all the requests, agencies declined to exercise march-in rights.
GAO was asked to review development of NIST’s draft guidance and its potential impacts. This report examines: (1) key elements of the draft guidance and the NIST-led interagency process for developing it; (2) stakeholder views on the draft guidance, as reflected in public comments; and (3) available information about the potential impacts of exercising march-in rights based on price.
GAO reviewed applicable laws and regulations, analyzed public comments and patent data, reviewed studies estimating how many drugs could be affected by exercising march-in rights based on price, and interviewed agency officials.
For more information, contact Candice N. Wright at wrightc@gao.gov.
