Antiviral Drugs: Economic Incentives and Strategies for Pandemic Preparedness [Reissued with revisions on Sept. 29, 2023]
Fast Facts
Scientists have predicted that another pandemic is likely. They've identified the viruses that might cause it—although the Department of Health and Human Services told us that few antiviral drugs for those viruses have been approved or are in HHS-funded clinical trials.
Our panel of experts suggested several ways for policymakers to incentivize the development of these antiviral drugs, such as research grants or commitments to buy the drugs once they're approved.
This report covers incentives and policy options to help develop antiviral drugs now, should policymakers want to invest in these drugs to prepare for a future pandemic.

The report distributed the morning of September 29th, 2023 was revised to list congressional committees as the addressees on the highlights page.
Highlights
What GAO Found
Antiviral drugs help the body fight off harmful viruses and can ease symptoms and shorten the length of an infection. Some of these drugs can be broadly acting—that is, they can treat infections from multiple viruses. This makes them especially valuable in a future pandemic where the viral threat is unknown in advance. Some scientists predict that viruses are most likely to be the source of the next pandemic because they are able to spread rapidly and because there are fewer treatment options for viruses.
The National Institutes of Health (NIH) has identified several viral families that have potential to cause future pandemics. However, as of May 2023, no known drugs for a number of these viral families had been approved or were in clinical trials funded by the Department of Health and Human Services (HHS), according to HHS. GAO identified a number of technologies to speed antiviral drug development, such as using artificial intelligence to identify drug candidates.
State of Drug Development for Viral Families with High Potential to Cause Future Pandemics
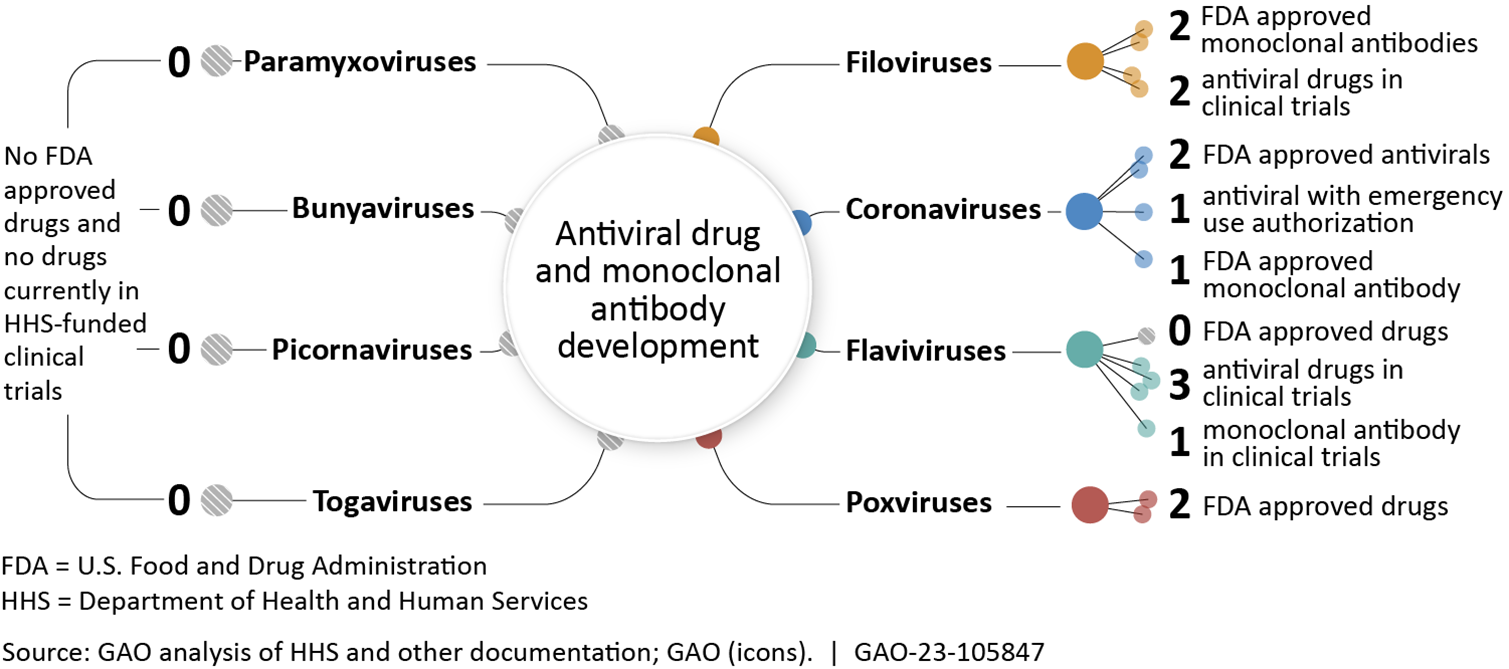
Note: Monoclonal antibodies are lab-produced proteins that can be used to treat or prevent viral infections. Graphic refers to clinical trials funded by either the Biomedical Advanced Research Development Authority or the National Institute of Allergy and Infectious Disease within the Department of Health and Human Services (HHS).
Experts GAO spoke with noted that a number of factors complicating the market’s efficient functioning make it unlikely that market forces alone will induce the development of antiviral drugs at levels that would maximally benefit society and aid future pandemic preparedness. However, policymakers could use several mechanisms to incentivize investment in antiviral drugs. “Push” mechanisms, such as research grants, can incentivize early research, while “pull” mechanisms, such as purchase commitments, can incentivize the production of completed antivirals.
In 2022, the White House issued the National Biodefense Strategy and Implementation Plan, which called for the development of two novel antiviral drugs. Experts GAO spoke with also identified a number of approaches to guide the use of economic incentives. These approaches include developing (1) a number of antiviral drugs to the point of phase 1 clinical trials, which could be less costly than developing fully approved drugs; (2) a wide range of antiviral drugs that act against different parts of the viral lifecycle; and (3) broadly acting antiviral drugs for a wide range of pathogens.
GAO identified three policy options that may help to spur antiviral drug development. These policy options are provided to inform policymakers—including Congress, federal agencies, state and local governments, academia, industry, and international organizations—about potential approaches and actions to address gaps highlighted in this technology assessment. Policymakers would need to consider how to align these potential actions with existing federal programs and initiatives. See below for a summary of policy options as well as related opportunities and considerations.
Policy Options to Facilitate the Development of Antiviral Drugs for Future Pandemics
| Policy option | Opportunities | Considerations |
|---|---|---|
|
Create a strategy to focus on developing diverse antiviral drugs to respond to pandemics caused by the most dangerous pathogens (report p. 33). Policymakers could ensure focus on pathogens and pathogen families, such as flaviviruses and bunyaviruses, that are most likely to cause severe future pandemics, with an emphasis on pathogen families for which no drug candidates are currently in clinical trials. |
|
|
|
Assign to a new or existing entity the authority to lead, implement, and be accountable for identifying and developing antiviral drugs for pathogens or pathogen families of greatest risk (report p. 34). Policymakers could ensure that this entity has authority and accountability for making decisions and allocating resources to implement a strategy for addressing pathogens and pathogen families informed by pandemic scenarios and the risks they present. |
|
|
|
Implement economic incentives to develop antiviral drug candidates and spur new drug-development technologies (report p. 35). Policymakers could use various push and pull economic incentives to encourage the development of pandemic antiviral drugs, based on the economic costs of scenarios, scientific opportunities, and pathogen priorities. They could also consider other policy priorities, such as drug accessibility. Policymakers could use economic incentives to stockpile certain drugs in advance as well to ensure sufficient manufacturing capacity. |
|
|
Source: GAO. | GAO-23-105847
Why GAO Did This Study
Pandemics impose large human and economic costs on society. According to the World Health Organization, almost 7 million people died worldwide during the COVID-19 pandemic. The International Monetary Fund estimates that by 2024, COVID-19 will have reduced global economic output by $13.8 trillion relative to prepandemic forecasts. Scientists have predicted that another pandemic is highly likely.
The CARES Act included a provision for GAO to report on the federal response to the COVID-19 pandemic. This report discusses incentives and strategies to enhance antiviral drug investment.
GAO reviewed economic and scientific literature relevant to these areas. GAO also interviewed stakeholders and experts with perspectives on the science and economics of antiviral drug development, including those at HHS. In addition, GAO convened, with assistance from the National Academies of Sciences, Engineering, and Medicine, a 2-day meeting of 16 experts.
GAO is identifying policy options in this report.
For more information, contact Michael Hoffman at (202) 512-2700, HoffmanME@gao.gov or Candice Wright at (202) 512-6888, WrightC@gao.gov.
