Defense Production Act: Opportunities Exist to Increase Transparency and Identify Future Actions to Mitigate Medical Supply Chain Issues:
Fast Facts
The Defense Production Act allows federal agencies to require companies to prioritize government contracts for medical supplies to address COVID-19. Agencies initially used the authority to address early medical supply shortages for ventilators and N95 respirators, and eventually for other critical supplies like masks and testing supplies.
Health and Human Services is leading an effort to address U.S. medical supply chain issues related to the pandemic, but hasn't clarified its plans. We recommended that HHS identify how it will use the Act to increase U.S. production of critical medical supplies and reduce reliance on foreign sources.

Highlights
What GAO Found
Federal agencies used the Defense Production Act (DPA) to help address medical supply shortages from COVID-19. The DPA gives agencies the authority to (1) prioritize contracts for medical supplies so those orders get preference over others, and (2) expand domestic production of medical supplies. GAO identified 43 contracts and agreements—initially valued at about $3.9 billion—where agencies placed priority ratings on or funded domestic production expansion projects for COVID-19 medical supplies (see figure). Department of Health and Human Services (HHS) and Federal Emergency Management Agency (FEMA) officials stated that nearly all the approximately 181,000 ventilators and 166.5 million of the respirators they placed on priority rated contracts, respectively, have been delivered as of September 2020.
Federal Agencies' Use of Defense Production Act and Similar Actions for Medical, Testing, and Vaccine Supplies, March 2020-September 2020
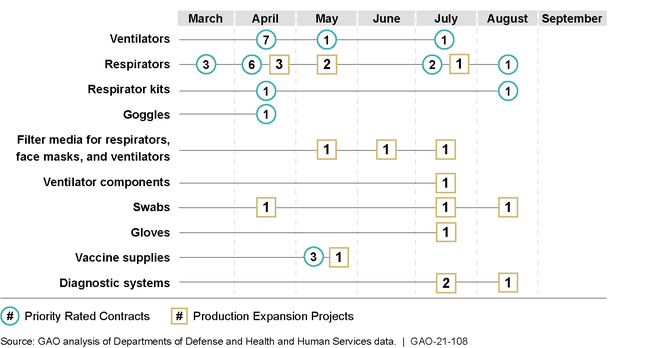
Note: One respirator contract included orders for powered air purifying respirator kits and goggles. We show these as three separate actions.
Federal agencies initially used a targeted DPA approach to address early COVID-19 medical supply issues, such as for ventilators and N95 respirators. By September 2020, agencies increased use of DPA and similar actions to a total of 10 types of medical supplies, and additional DPA actions are likely for masks, pharmaceuticals, screening and diagnostics, and personal protective equipment. In light of COVID-19 supply issues, an August 2020 executive order also directed that agencies take steps to reduce U.S. reliance on foreign manufacturers of medical supplies and other items. To support this order, HHS is leading an effort to identify and mitigate risks for increasing domestic production of medical supplies, but the agency has not yet identified its plans. As HHS completes this effort, an opportunity exists to identify how the DPA and similar actions may be needed to support the effort. Specifically, identifying further use of the DPA and similar actions to increase domestic production of key medical supplies can help alleviate national security risks from continued reliance on foreign manufacturers.
Why GAO Did This Study
COVID-19 has put the U.S. health care system under severe strain, including affecting the federal government's ability to buy and maintain critical medical supplies to treat patients and protect health care workers.
In March 2020 agencies began using DPA authorities to rapidly obtain and expand domestic production of medical supplies for COVID-19. The CARES Act provided the Department of Defense (DOD) $1 billion for DPA purchases related to COVID-19. HHS also reported using some of the $8.4 billion it obligated to buy supplies and replenish the Strategic National Stockpile to increase domestic production of medical supplies, which GAO refers to as similar actions.
The CARES Act includes a provision for GAO to monitor funds provided for the COVID-19 pandemic. This report examines (1) federal agencies' use of these actions to address COVID-19, and (2) the federal approach for using DPA and similar actions for medical supplies, among other issues. GAO analyzed agency announcements, federal procurement data, contracts, project data, and planning documents from March 18 through September 30, 2020, and interviewed HHS, DOD, and FEMA officials.
Recommendations
GAO is making two recommendations, including that HHS identify how DPA and similar actions will be used to increase domestic production of essential medical supplies as part of efforts to reduce reliance on foreign manufacturers. HHS concurred with the recommendation.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Office of Management and Budget |
Priority Rec.
OMB should direct the Office of Federal Procurement Policy to develop appropriate agency reporting guidance to provide greater transparency on the use of DPA Title I authorities for COVID purposes. The reporting guidance should enable taxpayers and other interested stakeholders to see where a priority rating was placed on the contract or contract modification for COVID-19 purposes. (Recommendation 1) |
OMB's Office of Federal Procurement Policy (OFPP)) agreed with this recommendation. In response, in July 2021 OFPP created a page in its MAX Information System, an Office of Management and Budget government-wide collaboration site, to collect information on past and future COVID-related DPA Title I awards. Agencies were notified that they had until July 30, 2021, to enter all past and current award information and were instructed to update the site at least quarterly afterwards with any additions. They were also notified that any entered information will be made public. The collection exercise was coordinated with DPA representatives at HHS and FEMA to leverage existing datasets and reduce burden.
|
| Office of the Assistant Secretary for Preparedness and Response |
Priority Rec.
The HHS Assistant Secretary for Preparedness and Response should identify how DPA and similar actions will be used to increase domestic production of medical supplies going forward. This could be included in HHS's 180-day effort to identify and mitigate vulnerabilities for essential medicines, medical countermeasures, and critical inputs that is required to support Executive Order 13944, which is aimed at reducing reliance on foreign manufacturers of medical supplies. (Recommendation 2) |
In March 2023 HHS responded that it has taken several steps to use DPA authorities to address medical supply chain risks. For example, HHS used DPA Title I authorities to issue 70 priority ratings for U.S. government contracts for health resources and industrial expansion, as well as non-U.S. government contracts to ensure private sector partners manufacturing medical supplies can acquire prerequisite raw materials, components, and products. HHS plans to establish a Title III Program in 2023 that will target investments to sustain production of critical medical supplies, commercialize research and development investments, and scale emergency technologies to enhance or expand domestic public health industrial base capabilities. HHS is also using $5.8 billion of American Rescue Plan funding to expand production of vaccine supplies and materials; personal protective equipment capacity and raw materials; and testing capacity and raw materials. These actions should better position HHS to help bolster domestic production of medical supplies and reduce U.S. dependence on foreign manufacturers of medical supplies.
|
