Preventing Drug Abuse: Low Participation by Pharmacies and Other Entities as Voluntary Collectors of Unused Prescription Drugs
Fast Facts
GAO-18-25
In 2015, 3.8 million Americans reported misusing prescriptions in the prior month, and deaths from prescription opioids have quadrupled since 1999. Most people get these drugs from friends or relatives, so providing secure and convenient ways for people to dispose of their unused drugs could help.
A 2010 federal law authorized pharmacies and other entities to voluntarily maintain a prescription drug disposal bin for the public. We found that 3% of entities eligible to collect drugs in this way volunteered to do so. Stakeholders reported that this is partly due to the cost of purchasing a bin and paying for the destruction of collected drugs.
Collecting Unused Prescription Drugs Using Disposal Bins
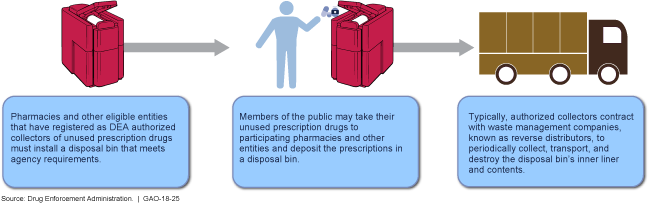
Flow chart showing how pharmacies and other eligible entities can collect and dispose of unused prescription drugs.
Highlights
What GAO Found
GAO found that about 3 percent of pharmacies and other entities eligible to collect unused prescription drugs for disposal have volunteered to do so. The Drug Enforcement Administration (DEA) authorizes these entities to dispose of unused drugs to help reduce their potential misuse. Analysis of DEA data shows that as of April 2017, 2,233 of the 89,550 (2.49 percent) eligible entities—that is, certain entities already authorized by DEA to handle controlled substances—had registered with DEA to use disposal bins to collect unused prescription drugs. Most—about 81 percent—of the authorized collectors were pharmacies, followed by hospitals or clinics. GAO also found that participation rates varied by state, though in 44 states less than 5 percent of the state's pharmacies and other eligible entities had registered to become authorized collectors.
Percentage of Eligible Entities Authorized by DEA to Collect Unused Prescription Drugs Using Disposal Bins, April 2017
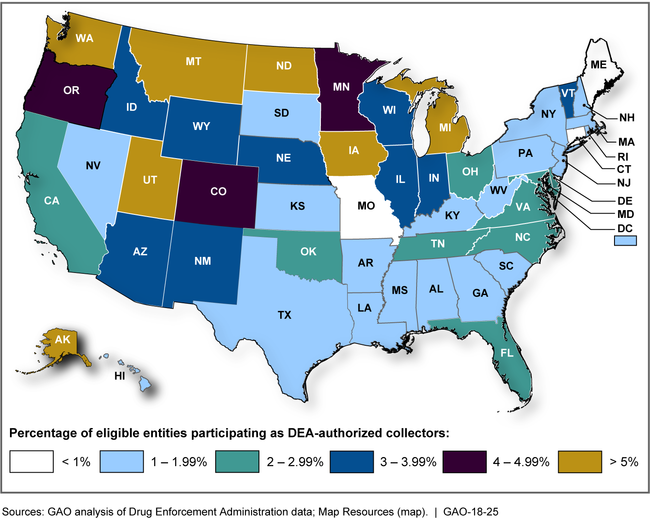
Stakeholders cited several factors that may explain why relatively few pharmacies and other eligible entities have registered with DEA as authorized collectors of unused drugs. Most notably, stakeholders representing authorized collectors told GAO that because participation is voluntary, the cost associated with maintaining a disposal bin—which includes purchasing and installing the bin according to DEA requirements and paying for the destruction of its contents—is an important factor to weigh against potential benefits. DEA noted that availability of disposal by law enforcement agencies also contributes to low participation.
Why GAO Did This Study
In 2015, 3.8 million Americans reported misusing prescription drugs within the last month, and deaths from prescription opioids have more than quadrupled since 1999. About half of the people who reported misusing prescription drugs in 2015 received them from a friend or relative.
One way to help prevent this kind of diversion and potential misuse is by providing secure and convenient ways to dispose of unused, unneeded, or expired prescription medications. The Secure and Responsible Drug Disposal Act of 2010 authorizes pharmacies and other entities already authorized by DEA to handle controlled substances to also collect unused prescription drugs for disposal. In 2014, DEA finalized regulations for the implementation of the Act, establishing a voluntary process for eligible entities to become authorized collectors of unused prescription drugs using disposal bins.
GAO was asked to review participation among authorized collectors that maintain disposal bins. In this report GAO describes (1) participation rates among entities eligible to collect unused prescription drugs and (2) factors that affect participation. GAO analyzed the most currently available DEA data from April 2017 on entities eligible to participate and those participating as authorized collectors. GAO also conducted interviews with DEA officials and a nongeneralizable sample of 11 stakeholder organizations selected to illustrate different types of authorized collectors and long-term care facilities. GAO is not making any recommendations. DEA provided technical comments, which GAO incorporated as appropriate.
For more information, contact Debra A. Draper at (202) 512-7114 or draperd@gao.gov.
