COVID-19: Critical Vaccine Distribution, Supply Chain, Program Integrity, and Other Challenges Require Focused Federal Attention
Fast Facts
This review of the federal response to the COVID-19 pandemic is our fifth comprehensive report since June 2020 about the implementation of the CARES Act.
We remain deeply troubled by the lack of sufficient federal action on critical gaps identified and by the lack of clear plans to address these gaps. For example, a clear and comprehensive vaccine distribution plan remains a work in progress.
As of January, 27 of our 31 previous recommendations had not been implemented. This report makes 13 new recommendations to improve agencies' public health and economic recovery efforts, including the development of a national testing strategy.
Highlights
What GAO Found
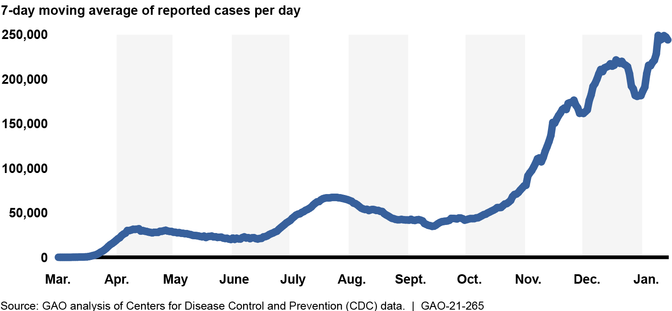
Since November 2020, the number of COVID-19 cases in the U.S. has rapidly increased, further straining health care systems across the country. Between December 31, 2020, and January 13, 2021, new reported COVID-19 cases averaged about 225,000 per day—over 7 and 3 times higher than the surges the nation experienced during the spring and summer of 2020, respectively. (See figure.) The country also continues to experience serious economic repercussions and turmoil as a result of the pandemic. As of December 2020, there were more than 10.7 million unemployed individuals, compared to nearly 5.8 million individuals at the beginning of the calendar year. Until the country better contains the spread of the virus, the pandemic will likely remain a significant obstacle to more robust economic activity.
In this report, GAO is making 13 recommendations to federal agencies to improve the ongoing response and recovery efforts in the areas of public health and the economy. As the new Congress and administration establish their policies and priorities for the federal government’s COVID-19 response, GAO urges swift action on these 13 recommendations, as well as on the additional recommendations that GAO has made since June 2020.
As of January 2021, 27 of GAO’s 31 previous recommendations remained unimplemented. GAO remains deeply troubled that agencies have not acted on recommendations to more fully address critical gaps in the medical supply chain. While GAO recognizes federal agencies continue to take some steps, GAO underscores the importance of developing a well-formulated plan to address critical gaps for the remainder of the pandemic, especially in light of the recent surge in cases. In addition, implementation of GAO’s recommendation concerning the importance of clear and comprehensive vaccine distribution and communication plans remains a work in progress. Moreover, slow implementation of GAO’s recommendations relating to program integrity, in particular those made to the Small Business Administration (SBA) and Department of Labor (DOL), creates risk of considerable improper payments, including those related to fraud, and falls far short of transparency and accountability expectations. See appendix III for the status of GAO’s past recommendations.
GAO is pleased that the Consolidated Appropriations Act, 2021—enacted in December of 2020—requires a number of actions that are consistent with several of GAO’s prior recommendations, including those related to the medical supply chain, vaccines and therapeutics, and COVID-19 testing. GAO will monitor the implementation of the act’s requirements.
GAO’s new recommendations are discussed below.
COVID-19 Testing
Diagnostic testing for COVID-19 is critical to controlling the spread of the virus, according to the Centers for Disease Control and Prevention. GAO found that the Department of Health and Human Services (HHS) has not issued a comprehensive and publicly available national testing strategy. HHS’s national strategy documents are not comprehensive because they only partially address the characteristics that GAO has found to be desirable in an effective national strategy. For example, testing strategy documents do not always provide consistent definitions and benchmarks to measure progress, not all documents clearly define the problem and risks, and there is limited information on the types of resources required for future needs.
Furthermore, some of the documents have not been made public. While the national testing strategy is formally outlined in a publicly available document, HHS has provided only Congress with the COVID-19 Testing Strategy Reports, which detail the implementation of the testing strategy. Stakeholders who are involved in the response efforts told GAO they were unaware of the existence of a national strategy or did not have a clear understanding of the strategy. Without a comprehensive, publicly available national strategy, HHS is at risk of key stakeholders and the public lacking crucial information to support an informed and coordinated testing response. GAO is recommending that HHS develop and make publicly available a comprehensive national COVID-19 testing strategy that incorporates all six characteristics of an effective national strategy. Such a strategy could build upon existing strategy documents that HHS has produced for the public and Congress to allow for a more coordinated pandemic testing approach. HHS partially concurred with this recommendation and agreed that it should take steps to more directly incorporate some of the elements of an effective national strategy.
Vaccines and Therapeutics
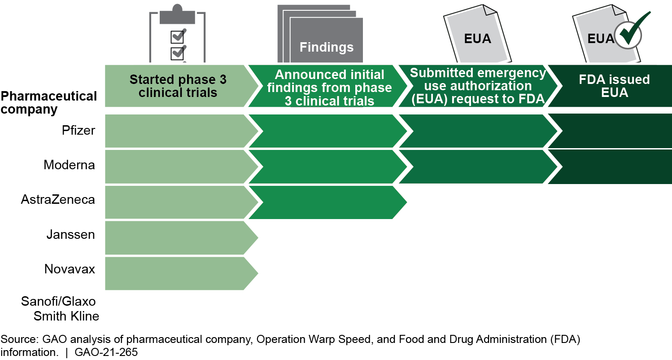
Multiple federal agencies, through Operation Warp Speed, continue to support the development and manufacturing of vaccines and therapeutics to prevent and treat COVID-19. As of January 8, 2021, two of the six vaccines supported by Operation Warp Speed have been authorized for emergency use, and vaccine distribution and administration have begun. (See figure below). However, distribution and administration fell short of expectations set for the end of the year. As of December 30, 2020, Operation Warp Speed had distributed (shipped) about 12.4 million doses of COVID-19 vaccine and providers reported administering about 2.8 million initial doses, according to Centers for Disease Control and Prevention data. In September 2020, GAO stressed the importance of having a plan that focused on coordination and communication and recommended that HHS, with the support of the Department of Defense, establish a time frame for documenting and sharing a national plan for distributing and administering COVID-19 vaccine, and among other things, outline an approach for how efforts would be coordinated across federal agencies and nonfederal entities.To date, this recommendationhas not been fully implemented. GAO reiterates the importance of doing so. Effective coordination and communication among federal agencies, commercial partners, jurisdictions, and providers is critical to successfully deploying COVID-19 vaccines and managing public expectations, especially because the initial supply of vaccine has been limited.
Medical Supply Chain
The pandemic has highlighted vulnerabilities in the nation’s medical supply chain, which includes personal protective equipment and other supplies necessary to treat individuals with COVID-19. The Strategic National Stockpile (SNS) is an important piece of HHS’s recently developed strategy to improve the medical supply chain to enhance pandemic response capabilities. However, the department has yet to develop a process for engaging about the strategy with key nonfederal stakeholders that have a shared role for providing supplies during a pandemic, such as state and territorial governments and the private sector. GAO’s work has noted the importance of directly and continuously involving key stakeholders, including Congress, in the development of successful agency reforms and helping to harness ideas, expertise, and resources.
To improve the nation’s response and preparedness for pandemics, GAO recommends that HHS establish a process for regularly engaging with Congress and nonfederal stakeholders—including state, local, tribal, and territorial governments and private industry—as the agency refines and implements its supply chain strategy for pandemic preparedness, to include the role of the SNS.HHS generally concurred with this recommendation and noted that the department regularly engages with Congress and nonfederal stakeholders. GAO maintains that capitalizing on existing relationships to engage these critical stakeholders as HHS refines and implements a supply chain strategy, to include the role of the SNS, will improve a whole-of-government response to, and preparedness for, pandemics.
In August 2020, the President issued an Executive Order directing agencies to take steps toward the goal of strengthening domestic drug manufacturing and supply chains. Federal agencies have started implementing the Executive Order, but expressed concerns about their ability to implement some of the provisions. In particular, GAO found that federal agencies do not have complete and accessible information to identify supply chain vulnerabilities and to report the manufacturing supply chains of drugs that were procured by the agency.
To help it identify and mitigate vulnerabilities in the U.S. drug supply chain, GAO recommends that the Food and Drug Administration (FDA) ensure drug manufacturing data obtained are complete and accessible, including by working with manufacturers and other federal agencies, such as the Department of Defense and the Department of Veterans Affairs and, if necessary, seek authority to obtain complete and accessible information. HHS neither agreed nor disagreed with this recommendation.
COVID-19 Data for Health Care Indicators
The federal government does not have a process to help systematically define and ensure the collection of standardized data across the relevant federal agencies and related stakeholders to help respond to COVID-19, communicate the status of the pandemic with citizens, or prepare for future pandemics. As a result, COVID-19 information that is collected and reported by states and other entities to the federal government is often incomplete and inconsistent. The lack of complete and consistent data limits HHS’s and others’ ability to monitor trends in the burden of the pandemic across states and regions, make informed comparisons between such areas, and assess the impact of public health actions to prevent and mitigate the spread of COVID-19. Further, incomplete and inconsistent data have limited HHS’s and others’ ability to prioritize the allocation of health resources in specific geographic areas or among certain populations most affected by the pandemic.
To improve the federal government’s response to COVID-19 and preparedness for future pandemics, GAO recommends that HHS immediately establish an expert committee comprised of knowledgeable health care professionals from the public and private sectors, academia, and nonprofits or use an existing one to systematically review and inform the alignment of ongoing data collection and reporting standards for key health indicators.HHS partially concurred with this recommendation and agreed that it should establish a dedicated working group or other mechanism with a focus on addressing COVID-19 data collection shortcomings.
Drug Manufacturing Inspections
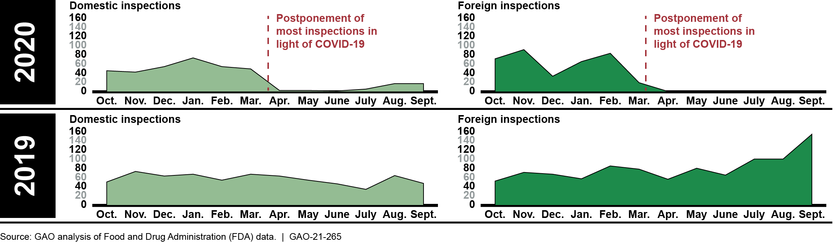
FDA is responsible for overseeing the safety and effectiveness of all drugs marketed in the U.S., including those manufactured overseas, and typically conducts more than 1,600 inspections of foreign and domestic drug manufacturing establishments every year. In light of the COVID-19 pandemic, since March 2020, FDA has limited domestic and foreign inspections for the safety of its employees. (See figure below.)
FDA has used alternative inspection tools to maintain some oversight of drug manufacturing quality while inspections are paused, including inspections conducted by foreign regulators, requesting and reviewing records and other information, and sampling and testing. Although FDA has determined that inspections conducted by certain European regulators are equivalent to an FDA inspection, other tools provide useful information but are not equivalent to an FDA inspection. As a result, FDA could be faced with a backlog of inspections, threatening the agency’s goal to maximize inspections prioritized by its risk-based site selection model each year.
GAO recommends that FDA (1) ensure that inspection plans for future fiscal years identify, analyze, and respond to the issues presented by the backlog of inspections that could jeopardize its goal of risk-driven inspections, and (2) fully assess the agency’s alternative inspection tools and consider whether these tools or others could provide the information needed to supplement regular inspection activities or help meet the agency’s drug oversight objectives when inspections are not possible in the future. FDA concurred with both recommendations.
Federal Contracting
Federal agencies are using other transaction agreements to respond to the pandemic, which are contracting mechanisms that can enable agencies to negotiate terms and conditions specific to a project. GAO found that HHS misreports its other transaction agreements related to COVID-19 as procurement contracts, including other transaction agreements with about $1.5 billion obligated for Operation Warp Speed and other medical countermeasures. HHS’s approach is inconsistent with federal acquisition regulations and limits the public’s insight into the agency’s contract spending. To ensure consistent tracking and transparency of federal contracting activity related to the pandemic, GAO recommends that HHS accurately report data in the federal procurement database system and provide information that would allow the public to distinguish between spending on other transaction agreements and procurement contracts.HHS concurred with this recommendation.
Oversight of Worker Safety and Health
GAO identified concerns about federal oversight of worker safety and health amid the COVID-19 pandemic. Specifically, the Occupational Safety and Health Administration (OSHA) has adapted its enforcement methods for COVID-19 to help protect agency employees from the virus and address resource constraints, such as by permitting remote inspections in place of on-site inspections of workplaces. However, gaps in OSHA’s oversight and tracking of its adapted enforcement methods prevent the agency from assessing the effectiveness of its enforcement methods during the pandemic, ensuring that its adapted enforcement methods do not miss violations, and ensuring that employers are addressing certain identified violations.
To improve its oversight, GAO recommends that OSHA (1) develop a plan, with time frames, to implement the agency’s oversight processes for COVID-19-adapted enforcement methods, and (2) ensure that its data system includes comprehensive information on use of these enforcement methods to inform these processes.The agency neither agreed nor disagreed with these recommendations.
Additionally, OSHA’s data do not include comprehensive information on workplace exposure to COVID-19. For example, OSHA does not receive employer reports of all work-related hospitalizations related to COVID-19, as disease symptoms do not appear within the required reporting time frames. Employers may also face challenges determining whether COVID-19 hospitalizations or fatalities are work-related because of COVID-19’s incubation period and the difficulties in tracking the source of exposure. GAO recommends that OSHA determine what additional datamay be neededfrom employers or other sources to better target the agency’s COVID-19 enforcement efforts.The agency neither agreed nor disagreed with this recommendation.
Assistance for Fishery Participants
The CARES Act appropriated $300 million in March 2020 to the Department of Commerce (Commerce) to assist eligible tribal, subsistence, commercial, and charter fishery participants affected by COVID-19, which may include direct relief payments. After administrative fees were assessed, $298 million of the $300 million appropriated was obligated for fishery participants.Widespread restaurant closures in the spring of 2020 led to a decrease in demand for seafood, adversely affecting the fisheries industry.
As of December 4, 2020, all funds had been obligated and only about 18 percent ($53.9 million) of the CARES Act funding obligated for fishery participants had been disbursed, which is inconsistent with Office of Management and Budget guidance on the importance of agencies distributing CARES Act funds in an expedient manner. Commerce’s National Oceanic and Atmospheric Administration (NOAA) officials said they expect that the vast majority of funds will be disbursed to fisheries participants by early 2021. However, the agency does not have the needed information centralized to help ensure that funds are being disbursed expeditiously and efficiently. GAO recommends that NOAA develop a mechanism to track the progress of states, tribes, and territories in meeting established timelines to disburse funds in an expedited and efficient manner. NOAA concurred with this recommendation.
Program Integrity
GAO continues to identify areas to improve program integrity and reduce the risk of improper payments for programs funded by the COVID-19 relief laws now that federal agencies have obligated a total of $1.9 trillion and expended $1.7 trillion of the $2.7 trillion appropriated for response and recovery efforts as of November 30, 2020. Federal relief programs remain vulnerable to significant risk of fraudulent activities because of the need to quickly provide funds and other assistance to those affected by COVID-19 and its economic effects.
In this report, GAO identifies concerns about overpayments and potential fraud in the unemployment insurance (UI) system, specifically in the federally funded Pandemic Unemployment Assistance (PUA) program, which provides UI benefits to individuals not otherwise eligible for these benefits, such as self-employed and certain gig economy workers. As of January 11, 2021, states that had submitted data to DOL reported more than $1.1 billion in PUA overpayments from March through December 2020. While DOL requires states to report data on PUA overpayments, as of the beginning of 2021, the agency was not tracking the amount of overpayments recovered, limiting insight into the effectiveness of states’ efforts to recoup federal funds. To better track the recovery of federal funds, GAO recommends that DOL collect data from states on the amount of PUA overpayments recovered. DOL concurred with this recommendation, and has taken the first step toward implementing it by issuing new guidance and updated instructions for states to report PUA overpayment recovery data.
GAO also remains concerned about SBA’s management of internal controls and fraud risks in the Economic Injury Disaster Loans (EIDL) program. COVID-19 relief laws made qualifying small businesses and nonprofit organizations adversely affected by COVID-19 eligible for financial assistance from the EIDL program. Some approval requirements were also relaxed, such as requiring each applicant to demonstrate that it could not obtain credit elsewhere, through December 31, 2021. As of December 31, 2020, SBA officials said they had approved about 3.7 million applications for loans related to COVID-19, totaling about $200 billion. SBA rapidly processed loans and advances to millions of small businesses affected by COVID-19. GAO’s analysis of SBA data shows that the agency approved EIDL loans and advances for potentially ineligible businesses. For example, SBA approved at least 3,000 loans totaling about $156 million to potentially ineligible businesses in industries that SBA policies state were ineligible for the EIDL program, such as insurance and real estate development, as of September 30, 2020. GAO recommends that SBA develop and implement portfolio-level data analytics across EIDL loans and advances made in response to COVID-19 as a means to detect potentially ineligible and fraudulent applications. SBA neither agreed nor disagreed with this recommendation.
Why GAO Did This Study
As of January 15, 2021, the U.S. had about 23 million cumulative reported cases of COVID-19 and more than 387,000 reported deaths, according to the Centers for Disease Control and Prevention. The country also continues to experience serious economic repercussions.
Four relief laws, including the CARES Act, were enacted as of November 2020 to provide appropriations to address the public health and economic threats posed by COVID-19. As of November 30, 2020, of the $2.7 trillion appropriated by these four laws, the federal government had obligated a total of $1.9 trillion and expended $1.7 trillion of the COVID-19 relief funds, as reported by federal agencies.
In December 2020, the Consolidated Appropriations Act, 2021, provided additional federal assistance for the ongoing response and recovery.
The CARES Act includes a provision for GAO to report on its ongoing monitoring and oversight efforts related to the COVID-19 pandemic. This report examines the federal government’s continued efforts to respond to and recover from the COVID-19 pandemic.
GAO reviewed data, documents, and guidance from federal agencies about their activities and interviewed federal and state officials and stakeholders. GAO completed its audit work on January 15, 2021.
Recommendations
GAO is making 13 new recommendations for agencies that are detailed in this Highlights and in the report.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Office of the Assistant Secretary for Preparedness and Response | To improve the nation's response to and preparedness for pandemics, the Assistant Secretary for Preparedness and Response should establish a process for regularly engaging with Congress and nonfederal stakeholders—including state, local, tribal, and territorial governments and private industry—as the Department of Health and Human Services refines and implements a supply chain strategy for pandemic preparedness, to include the role of the Strategic National Stockpile. (Recommendation 1) |
HHS generally concurred with our recommendation, and the Department has taken several actions that collectively meet its intent. For example, in August 2024, HHS finalized a strategic planning document for the Strategic National Stockpile (SNS) entitled "Strategy to Optimize the Strategic National Stockpile." The strategy identifies goals and action items related to (1) communicating SNS inventory gaps to Congress; (2) improving communication on SNS assets with state, local, tribal, and territorial (SLTT) stakeholders; and (3) coordinating with industry partners on medical supply availability. ASPR has augmented the strategy by taking other specific actions including modifying its SNS Medical Countermeasure Preparedness Review-the document that guides SNS inventory decisions-in May 2023 to better communicate inventory risks to Congress, establishing the Office of SLTT Preparedness in 2022 and holding outreach meetings with SLTT officials across the country throughout 2023, holding several briefings with Congressional staff in February and March 2023, introducing IBx Connect in February 2023 to work with industry on solutions to vulnerabilities in the medical supply chain, and continuing to monitor supply availability through the Supply Chain Control Tower. These activities collectively address the intent of our recommendation.
|
| Food and Drug Administration | The Commissioner of the Food and Drug Administration should, as the agency makes changes to its collection of drug manufacturing data, ensure the information obtained is complete and accessible to help it identify and mitigate supply chain vulnerabilities, including by working with manufacturers and other federal agencies (e.g., the Departments of Defense and Veterans Affairs), and, if necessary, seek authority to obtain complete and accessible information. (Recommendation 2) |
HHS neither agreed nor disagreed with our recommendation. In HHS's response in January 2021, FDA said that it will consider our recommendation as it continues efforts to enhance relevant authorities and close data gaps. Since then, FDA has taken some steps towards implementing our recommendation. For example, the agency issued guidance in February 2024 advising manufacturers on reporting the amount of each drug they manufacture for commercial distribution, which was required by the Coronavirus Aid, Relief, and Economic Security Act in 2020. In April 2025 (GAO-25-107110), we reported that, as of September 30, 2024, 44 percent of manufacturers of prescription drugs and 26 percent of over-the-counter drug manufacturers had submitted data on these amounts. FDA officials said they are unable to use the data collected until more manufacturers report and the agency is discussing ways to increase compliance. In addition, FDA officials also said these data will have gaps, such as not including the source of active pharmaceutical ingredients. Given these concerns, through its congressional budget justification for fiscal year 2025, FDA sought legislative authority to require enhanced reporting of supply chain data, such as information on manufacturers' suppliers and the extent to which the suppliers are relied on. These data are intended to address some of the gaps that the agency identified with the data that it is currently receiving. Utilizing the data its collecting and seeking additional authority would be consistent with our recommendation. We are monitoring FDA's collection and use of these data and will assess whether FDA's actions satisfy the intent of our recommendation.
|
| Department of Health and Human Services |
Priority Rec.
The Secretary of Health and Human Services should develop and make publicly available a comprehensive national COVID-19 testing strategy that incorporates all six characteristics of an effective national strategy. Such a strategy could build upon existing strategy documents that the Department of Health and Human Services has produced for the public and Congress to allow for a more coordinated pandemic testing approach. (Recommendation 3) |
HHS partially agreed with this recommendation. However, as of April 2024, this recommendation remains unimplemented. The recommendation remained valid during the public health emergency, and we maintained it was important to take action immediately. According to CDC, while COVID-19 still currently exists within the population today, it has become endemic. Furthermore, conditions continue to change now with the circulation of other infectious diseases, such as mpox and highly pathogenic avian influenza. In light of this, we no longer believe that HHS should engage in additional efforts to develop a testing strategy exclusive to COVID-19. For these reasons, GAO has decided to close this recommendation as no longer valid.
|
| Food and Drug Administration |
Priority Rec.
The Commissioner of the Food and Drug Administration should, as inspection plans for future fiscal years are developed, ensure that such plans identify, analyze, and respond to the issues presented by the backlog of inspections that could jeopardize the goal of risk-driven inspections. (Recommendation 4) |
FDA concurred with our recommendation. In June 2022,FDA reported that, to largely address the backlog within 3 years, it intended to: request and review records from establishments that have never been inspected to expedite inspections of such establishments and potentially remove them from the list of establishments in need of inspection; further identify and prioritize establishments that have not been inspected within 5 years and select a subset of this group to be mandatory for inspection in a given year; and conduct surveillance inspections at certain establishments to which FDA was already planning to go to conduct other types of inspections. FDA's inspection planning documents for fiscal years 2023 and 2024 reflect this planned approach to respond to the backlog by identifying the highest priority sites for inspection and using alternative approaches to maximize the use of surveillance inspection resources. Additionally, the fiscal year 2024 inspection planning document includes FDA plans to conduct abbreviated inspections at a subset of lower-risk sites in the backlog, allowing more sites to be inspected in total, and includes metrics to analyze the use of such abbreviated inspections. The planning documents present these changes as being intended to help increase capacity for risk-driven surveillance inspections, thereby responding to the issues presented by the backlog. For fiscal year 2024, the proportion of its planned surveillance inspections that are risk-driven has increased for the first time since the COVID-19 pandemic began, in alignment with FDA's goal. FDA's actions as of February 2024 meet the intent of our recommendation to identify, analyze, and respond to the issues presented by the backlog of inspections that could jeopardize the goal of risk-driven inspections.
|
| Food and Drug Administration | The Commissioner of the Food and Drug Administration should fully assess the agency's alternative inspection tools and consider whether these tools or others could provide the information needed to supplement regular inspection activities or help meet its drug oversight objectives when inspections are not possible in the future. (Recommendation 5) |
In June 2022, FDA reported that it had established a workgroup to fully assess the agency's practices regarding remote regulatory assessments and has continued to issue clarifying guidance on their use. FDA provided documentation related to the workgroup's assessment in June 2024. Based on the workgroup's assessment, in October 2023 and January 2024, FDA issued updated versions of previous guidance about the agency's use of remote assessments to clarify their post-pandemic relevance. As part of our work examining FDA's foreign drug inspection program and its use of alternative tools, officials described future assessments related to a proposal to request records in advance of certain inspections and to an ongoing collaborative hybrid inspections pilot. To fully implement this recommendation, FDA should complete these two planned assessments and provide them to GAO so we can determine if they meet the intent of our recommendation. As of February 2025, FDA did not have updates to this recommendation.
|
| Department of Health and Human Services |
Priority Rec.
To improve the federal government's response to COVID-19 and preparedness for future pandemics, the Secretary of Health and Human Services should immediately establish an expert committee or use an existing one to systematically review and inform the alignment of ongoing data collection and reporting standards for key health indicators. This committee should include a broad representation of knowledgeable health care professionals from the public and private sectors, academia, and nonprofits. (Recommendation 6) |
HHS partially agreed with our recommendation. HHS has taken steps to involve external stakeholders to help inform the alignment of COVID-19 data collection and reporting standards and to prepare for future pandemics, according to documents it provided us in April 2023. HHS used an existing committee comprised of federal agency and nonfederal members (the Health Information Technology Advisory Committee) to conduct a retrospective review of COVID-19 data collection and reporting standards and make recommendations to HHS to support future public health emergency responses. In addition, its Interagency Hospital Data Surveillance Council-which coordinates ongoing COVID-19 hospital data collection in hospitals-will be used to help with future pandemics by engaging with internal and external stakeholders to solicit feedback and ensure that hospital data collection aligns with the information needs and capabilities at state and other levels. Further, HHS established a long-term governance structure to provide ongoing guidance on data requirements and collection while considering end users and contributors of such data (e.g., federal components, state, and local health departments). The governance structure should consult external stakeholders such as national associations and councils representing state public health officials and hospitals for their input. We consider HHS's actions to meet the intent of our recommendation and therefore we are closing this recommendation as implemented.
|
| Office of the Assistant Secretary for Preparedness and Response | The Assistant Secretary for Preparedness and Response, in coordination with the appropriate offices within the Department of Health and Human Services, should accurately report data in the federal procurement database system and provide information that would allow the public to distinguish between spending on other transaction agreements and procurement contracts. (Recommendation 7) |
ASPR agreed with our recommendation. In October 2022, ASPR updated its contract writing system to enable the identification of other transaction agreements. While this update allows ASPR to better track its use of other transaction agreements internally, as of February 2025, ASPR's publicly available data in the federal procurement database system still does not distinguish between other transaction agreements and procurement contracts. ASPR officials stated they are exploring options to report to the federal procurement data system. We will continue to monitor ASPR's efforts to implement our recommendation.
|
| Occupational Safety and Health Administration |
Priority Rec.
The Assistant Secretary of Labor for Occupational Safety and Health should develop a plan, with time frames, to implement the agency's oversight processes for COVID-19-adapted enforcement methods, as described in its pandemic enforcement policies. (Recommendation 8) |
The Department of Labor (DOL) neither agreed nor disagreed with our recommendation. In May 2021 and December 2021, Occupational Safety and Health Administration (OSHA) officials said that the agency was no longer planning to conduct the oversight outlined in its April and May 2020 pandemic-related enforcement policies, which provided guidance to inspectors for COVID-19-related enforcement until the March 2021 pandemic-related enforcement policy was issued. For oversight of remote inspections, in May 2021, officials said that follow-up for some, but not all, remotely-conducted inspections would be performed according to area offices' discretion as part of OSHA's COVID-19 National Emphasis Program (NEP), as resources permit area offices to focus more on programmed inspections. For oversight of informal inquiries conducted in place of inspections, in December 2021, officials said that follow-up inspections could only be conducted when an initial inspection had occurred, not when an initial informal inquiry had occurred. Instead, officials said the agency plans to conduct an overall assessment of its efforts during the pandemic once the pandemic is no longer impacting OSHA's enforcement activities, and that this assessment will include an analysis of the agency's use of remote inspections and informal inquiries. For oversight of citation discretion, in December 2021, officials said that they had reviewed the six instances of citation discretion documented in the OSHA Information System (OIS). They confirmed that hazard abatement occurred in five instances, and planned a follow-up inspection for the sixth. Officials noted that, although they are not able to track whether citation discretion was used but not documented in OIS, there has been no indication that this is of any significant concern.
|
| Occupational Safety and Health Administration |
Priority Rec.
The Assistant Secretary of Labor for Occupational Safety and Health should ensure that the Occupational Safety and Health Administration Information System includes comprehensive information on use of the agency's COVID-19-adapted enforcement methods sufficient to inform its oversight processes for these methods. (Recommendation 9) |
The Department of Labor (DOL) neither agreed nor disagreed with our recommendation. Throughout the pandemic, the Occupational Safety and Health Administration (OSHA) made several OSHA Information System (OIS) updates to better track the agency's use of COVID-19-adapted enforcement methods, including adding OIS codes to identify inspections conducted remotely and inspections related to COVID-19. OIS continues to be unable to systematically differentiate between typical informal inquiries and informal inquiries used in place of inspections due to pandemic-related constraints. In December 2021, officials stated that any notes regarding an informal inquiry being used in place of an inspection would be part of the OIS case file, which could be reviewed individually, and that although there is no automated report available, they do not believe that impedes OSHA's ability to conduct COVID-19-related enforcement. Because OSHA is no longer planning to conduct its planned oversight of informal inquiries used in place of inspections, the need to systematically identify this subset of all informal inquiries is no longer essential. For citation discretion, in December 2021, officials said that they completed their review of the six instances of citation discretion recorded in OIS and corrected any instances of the relevant OIS code being entered improperly. Officials noted that, although they are not able to track whether citation discretion was used but not documented in OIS, there has been no indication that this is of any significant concern. OSHA generally ended use of pandemic-related citation discretion in July 2021. In December 2021, OSHA officials said that OSHA will assess what changes, if any, should be made to OIS during the post-pandemic assessment that OSHA will conduct.
|
| Occupational Safety and Health Administration | The Assistant Secretary of Labor for Occupational Safety and Health should determine what additional data may be needed from employers or other sources to better target the agency's COVID-19 enforcement efforts. (Recommendation 10) |
In February 2021, OSHA said that, in response to our recommendation, it had determined that it did not need additional information from employers to identify where pandemic-related enforcement should be targeted. However, OSHA's June 2021 health-care emergency temporary standard (ETS) specifically addressed the data gap that we identified in January 2021 related to employer reporting of COVID-19-related hospitalizations for certain health-care employers whose employees, OSHA determined, face "grave danger". OSHA therefore did determine that it needed additional data from certain employers for its enforcement efforts, in accordance with our recommendation.
|
| National Marine Fisheries Service | The Assistant Administrator for the National Oceanic and Atmospheric Administration Fisheries should develop a mechanism to track the progress of states, tribes, and territories in meeting timelines established in spend plans to disburse funds in an expedited and efficient manner. (Recommendation 11) |
In February 2021, NOAA developed an electronic tracking tool to track the disbursement of funds to fisheries participants, and as of July 2021, the agency was regularly inputting data into this tool to monitor the progress of states, tribes, and territories to disburse funds.
|
| Office of Unemployment Insurance | The Secretary of Labor should ensure the Office of Unemployment Insurance collects data from states on the amount of overpayments recovered in the Pandemic Unemployment Assistance program, similar to the regular unemployment insurance program. (Recommendation 12) |
DOL agreed with our recommendation and on January 8, 2021, issued PUA program guidance and updated instructions for states to report PUA overpayments recovered. As of June 2023, all 53 states and territories have reported some data on the amount of PUA overpayments recovered in any month, though some have reported zero amounts recovered. DOL will continue to monitor all states' and territories' data to ensure that their reporting of PUA overpayments recovered is accurate.
|
| Small Business Administration |
Priority Rec.
The Administrator of the Small Business Administration should develop and implement portfolio-level data analytics across Economic Injury Disaster Loan program loans and advances made in response to COVID-19 as a means to detect potentially ineligible and fraudulent applications. (Recommendation 13) |
At the time of our report, SBA neither agreed nor disagreed with this recommendation. In response, in December 2021, SBA stated that the agency had implemented fraud indicators for EIDL application data and had shared these indicators with the Pandemic Response Accountability Committee for review. In June 2022, SBA officials provided a demonstration of an EIDL data analytics/loan anomaly detection project that the agency had initiated. In September 2022, SBA provided reports and log files related to the foreign area code and email address shifting period tests, as examples of EIDL analytics tests that had been run to detect potential fraud.
|
