340B Drug Discount Program: Agency Oversight Has Improved, but Actions Needed to Address Weaknesses
Fast Facts
We testified on the 340B Drug Discount Program before the U.S. Senate Committee on Health, Education, Labor & Pensions.
Our statement is based primarily on the following reports:
Drug Discount Program: Federal Oversight of Compliance at 340B Contract Pharmacies Needs Improvement
We have made numerous recommendations related to 340B Program oversight to the Health Resources and Services Administration (HRSA), within the Department of Health and Human Services, which oversees the program.
HRSA has addressed some of our recommendations, but most remain unimplemented.

A picture of the U.S. Capitol with the text "GAO: Testimony to Congress."
Highlights
What GAO Found
The 340B Drug Pricing Program (340B Program) requires drug manufacturers to sell outpatient drugs at discounted prices to covered entities—certain federal grantees and hospitals—to have their drugs covered by Medicaid. In the 10-year period between 2013 and 2023—when we last reported on the program—the number of covered entity sites more than doubled.
340B Program Covered Entity Sites by Type, 2013 and 2023
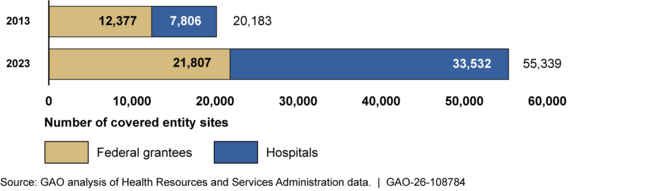
Note: Numbers are as of January 1 of each year and represent the number of covered entities and associated sites.
GAO has identified numerous weaknesses in the Health Resources and Services Administration’s (HRSA) oversight of the 340B Program. HRSA has taken steps to address some of these weaknesses, including implementing five of 20 GAO recommendations. Most notably, in fiscal year 2012, in response to a GAO recommendation, HRSA implemented a systematic approach to auditing covered entities and now audits 200 covered entities a year. Over time, HRSA has made other changes to strengthen its oversight by establishing an annual recertification process and other program integrity checks.
However, other weaknesses that GAO identified in HRSA’s audits and oversight remain unaddressed. For example:
- HRSA’s process for closing audits does not ensure covered entities have fully addressed any noncompliance identified.
- HRSA’s audits do not fully assess compliance with the program requirement that prohibits covered entities from subjecting manufacturers to duplicate discounts, in which drugs are subject to 340B discounted prices and rebates under the Medicaid program.
- HRSA’s oversight does not ensure only eligible hospitals participate in the program.
HRSA did not concur with six of the 15 unimplemented recommendations GAO made to address weaknesses in HRSA’s oversight. HRSA concurred with the remaining nine recommendations, but the agency has expressed concerns that it lacks the necessary enforcement capability to implement some of them. HRSA has requested that Congress provide it with additional regulatory authority for the 340B Program.
Why GAO Did This Study
Covered entities can realize substantial savings through 340B Program price discounts and, according to HRSA, these savings can enable them to stretch federal resources to reach more eligible patients and provide more comprehensive services. Covered entities can provide 340B drugs to eligible patients regardless of income or insurance status and can generate revenue under the program when insurance reimbursement exceeds the 340B price paid for the drugs.
HRSA is responsible for administering the program and overseeing covered entities’ compliance with program requirements. Program requirements include that covered entities must (1) prevent diversion of 340B drugs to individuals who are not eligible patients of the covered entities, and (2) avoid subjecting manufacturers to duplicate discounts.
This statement provides an overview of GAO’s assessment of HRSA’s oversight of the 340B Program.
This statement is primarily based on four GAO reports issued from 2011 through 2020 and on steps HRSA has taken to address GAO recommendations from those reports, as of February 2025. See GAO-11-836, GAO-18-480, GAO-20-108, and GAO-20-212. Those reports provide further details on our scope and methodology.
For more information, contact Michelle B. Rosenberg at RosenbergM@gao.gov.
