Human Organ-On-A-Chip: Technologies Offer Benefits Over Animal Testing but Challenges Limit Wider Adoption
Fast Facts
We examined "human organ-on-a-chip" technologies—laboratory tools that use human cells to mimic organs, model diseases, and more.
This new technology can complement conventional lab methods, such as animal testing, which can be inefficient at predicting human responses to drugs and other therapies.
While promising, this emerging technology faces challenges such as:
Difficulty getting enough high-quality human cells
Lack of benchmarks and validation studies
Little data sharing between competing companies
Unclear federal guidance on how the tech can meet regulatory requirements
We offer policy options to address these and other challenges.
Organ-on-a-chip in a researcher’s hands
Highlights
What GAO Found
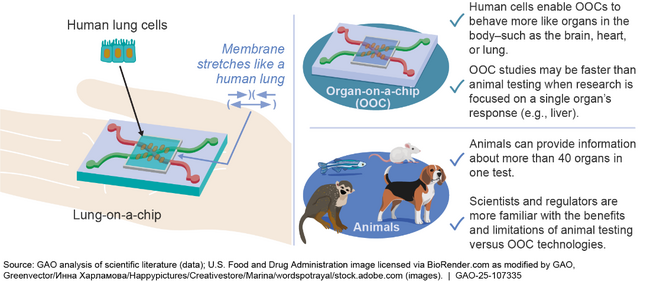
Human organ-on-a-chip (OOC) is an emerging technology that incorporates human adult cells in a small laboratory device to mimic how organs work—such as the brain, heart, or lungs. OOCs may be designed to simulate the mechanics of human organs, such as a lung-on-a-chip that stretches to simulate breathing. Researchers are developing and using OOCs to model diseases and predict responses to chemicals. For example, companies are using OOCs to assess aspects of drug safety and efficacy. Because OOCs contain human cells, their use in research may have more relevance to humans than animal testing or other conventional lab methods, but certain OOC research may cost more and take longer. Currently, OOCs cannot replace animal testing but may be used alongside animals. The next generation of OOC is focused on developing “body-on-a-chip” systems that link together multiple OOCs—such as the intestine, liver, and kidney—to investigate how organs interact.
Lung-on-a-chip (left) and how organ-on-a-chip compares to animals (right)
GAO identified several challenges to the development and use of OOCs, including
Obtaining human cells. Reliable human cells are a key requirement for using OOCs, but availability is limited. For example, experts told GAO that only 10 percent to 20 percent of human cells they purchase are high enough quality for OOC studies.
Lack of benchmarks and validation studies. The lack of benchmarks and sufficient studies assessing OOC accuracy, reliability, and relevance hinders the ability of end users, such as drug companies, to understand how OOCs compare to conventional methods, including animals, and data from clinical trials.
Limited data sharing. OOC developers and end users may be reluctant or lack capacity to share their OOC research findings. For example, companies may be concerned about intellectual property and loss of competitive advantages.
Regulatory uncertainties. Regulators are still working to better understand OOCs. Meanwhile, experts told GAO that the OOC field faces regulatory uncertainties, including regulators’ lower level of familiarity with OOCs than other methods, and unclear guidance and messaging from the U.S. Food and Drug Administration.
GAO developed six policy options that could help mitigate challenges to OOC development and use, including the option to maintain the status quo. The options identify possible actions by policymakers, including legislative bodies, government entities, academia, industry, and other groups. See tables 1–6 in this report for additional policy options and details.
Selected Policy Options to Help Mitigate Challenges to the Development and Use of Organ-on-a-Chip (OOC) Technologies
| Selected policy option | Opportunities | Considerations |
|---|---|---|
|
Support efforts to increase access to diverse, high-quality human cells (report p. 25)
For example, federal entities, together with stakeholders from academia and industry, could support the establishment of high-quality cell banks and biospecimen repositories that incorporate population diversity. |
|
|
|
Encourage more research and development of benchmarks and validation studies (report p. 27)
For example, relevant funding agencies could provide more funding to academics and companies for OOC research, specifically to validate OOCs for priority contexts of use. |
|
|
|
Create or participate in mechanisms for data sharing (report p. 28)
For example, OOC developers and drug companies could participate in precompetitive efforts to share OOC data freely, such as sharing data through an industry trade group, nonprofit, or other trusted third party. |
|
|
|
Provide additional regulatory guidance (report p. 29)
For example, regulators could provide detailed guidance on how specific OOCs could more readily replace a conventional laboratory method. |
|
|
Source: GAO. | GAO-25-107335
Why GAO Did This Study
Biomedical researchers face challenges investigating human biology, disease, and the body’s responses to external chemicals, including medicines. Conventional methods—such as animal testing and cells in a petri dish—often do not translate into similar results for humans because they cannot effectively replicate the complex systems of the human body. In the past decade, however, researchers in the pharmaceutical, chemical, and biodefense industries have begun developing and using OOCs to help improve our understanding of human disease and responses to drugs and other chemicals.
This technology assessment examines (1) current and emerging OOC technologies and their potential benefits, (2) challenges to developing and using these technologies, and (3) options that policymakers could consider to help enhance the benefits or mitigate the challenges.
To conduct this work, GAO reviewed scientific literature and federal agency documents; interviewed a range of experts from government, industry, academia, and nonprofit organizations; and convened a discussion group of 16 experts. Participants included federal agency officials, policy experts, and OOC developers and end users from industry and academia. GAO is identifying policy options in this report (see next page).
For more information, contact Karen L. Howard, PhD, at HowardK@gao.gov.
