Food Safety: FDA Should Finalize Plans to Implement Its Rule to Help Trace Source of Outbreaks
Fast Facts
Although the U.S. food supply is generally considered safe, foodborne illness is a common public health problem. Certain foods—like fresh produce and seafood—have often been linked to foodborne illness outbreaks.
The Food and Drug Administration has a new rule requiring detailed records on certain foods as they move through the supply chain, which can help trace the sources of outbreaks. FDA has taken some steps—such as issuing guidance—to help implement the rule. We recommended that FDA finalize its plans for implementing the rule to help industry and regulators prepare for compliance by January 20, 2026.
Food safety is on our High Risk list.

Highlights
What GAO Found
In November 2022, the Food and Drug Administration (FDA) promulgated a final rule on food traceability to help identify the source of outbreaks of foodborne illness. In developing the rule, the FDA established a list of certain foods for which enhanced recordkeeping is required, and set a compliance date of January 20, 2026. Entities handling an item on the list must maintain specific records, including a traceability plan, at certain points in the item's supply chain.
To identify foods for the list, FDA used an approach that incorporates statutorily mandated criteria, such as the history and severity of prior outbreaks involving the item. Several stakeholders GAO interviewed said FDA's methodology for identifying foods for the list was appropriate. Several other stakeholders disagreed with this assessment, stating that FDA's approach resulted in an overly inclusive list. In response to similar comments on the draft rule, FDA provided its rationale for considering foods at the commodity—or category—level, stating that foods in these groups had similar risk characteristics and associated hazards.
Examples of Points in the Supply Chain Required to Maintain Traceability Records for a Produce Item on the Food Traceability List
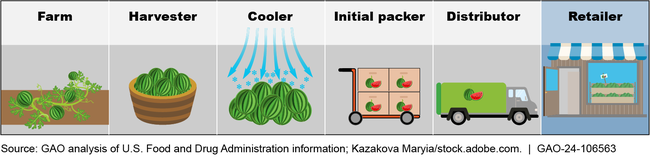
FDA has taken some steps to help industry and nonfederal regulators prepare for compliance with and enforcement of the rule. Also, in late 2022, FDA began an iterative planning process for implementing the rule. However, as of October 2023, FDA had not finalized or documented an implementation plan, according to FDA officials.
Components of such a plan could help address challenges stakeholders identified in preparing for the compliance deadline. For example, the plan could include additional information on nonfederal regulators' roles in the inspection process and FDA's enforcement strategy and needed resources. It also could identify additional guidance, training, and tools for stakeholders. By finalizing and documenting an implementation plan, FDA will have better assurance it is well positioned to make progress toward its regulatory goals and address the various challenges that stakeholders identified to achieving compliance by the deadline.
Why GAO Did This Study
Foodborne illness remains a common and costly public health problem in the U.S. Being able to efficiently trace products linked to a foodborne illness outbreak can help government agencies and those who produce and sell food identify the source of the outbreak. FDA, within the U.S. Department of Health and Human Services (HHS), is responsible for developing and implementing several rules required by the FDA Food Safety Modernization Act, enacted in 2011. These include the food traceability rule.
The act also included a provision for GAO to report on the traceability rule. This report, among other things, (1) describes FDA's and selected stakeholders' views on the rule's recordkeeping requirements and (2) examines FDA's actions to implement the rule and challenges FDA and stakeholders may face in achieving compliance.
GAO reviewed FDA documentation and interviewed FDA officials and 20 selected stakeholders representing industry associations, consumer advocacy groups, and nonfederal regulators.
Recommendations
GAO recommends that FDA finalize and document an implementation plan for the traceability rule. HHS agreed with this recommendation.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Food and Drug Administration | The FDA Commissioner should direct the Center for Food Safety and Applied Nutrition to finalize and document an implementation plan to help the agency achieve its regulatory goal of compliance with the food traceability rule by January 20, 2026. Such a plan should include FDA's resource needs, strategies for facilitating compliance with the rule, and detailed plans for communicating with and educating regulated entities, nonfederal regulatory partners, and FDA regulatory staff about the rule's requirements. (Recommendation 1) |
FDA agreed with this recommendation. In March 2025, FDA provided information on steps the agency has taken towards drafting its implementation plan, such as developing an inspectional framework and an initial assessment of resource needs. However, HHS did not provide a timeline related to FDA's efforts to finalize the agency's implementation plan. FDA later announced in March 2025 its intention to extend the compliance date for the rule by 30 months, or until July 2028. We will continue to monitor the status of the recommendation and the agency's actions to implement it.
|
