Antibiotic Resistance: Federal Agencies Have Taken Steps to Combat the Threat, but Additional Actions Needed
Fast Facts
Antibiotic-resistant infections can be difficult or impossible to treat. This testimony covers our work on federal efforts to address the following challenges:
Surveillance—CDC doesn't have enough data on antibiotic-resistant infections in health care settings
Diagnostics—More studies are needed to help develop and promote the use of tests to diagnose resistance
New treatments—The pipeline of new antibiotics is inadequate and not fully federally incentivized
Antibiotic use—Federal antibiotic-use requirements only apply to hospitals and nursing homes
We also describe actions federal agencies have taken to address our prior recommendations.
Highlights
What GAO Found
Bacterial infections have become more difficult, and sometimes impossible, to treat, as some have become resistant to antibiotics. However, the precise magnitude of this problem is unknown. GAO found in March 2020 that the Department of Health and Human Services (HHS) had made progress expanding surveillance of antibiotic-resistant bacterial infections, but faced data and other challenges. For example, to monitor resistant gonorrhea infections, HHS only tracked an estimated 1 to 2 percent of all U.S. cases, and only in males.
2001-2017 Cumulative Spread of One Type of Highly Resistant Bacteria in the United States
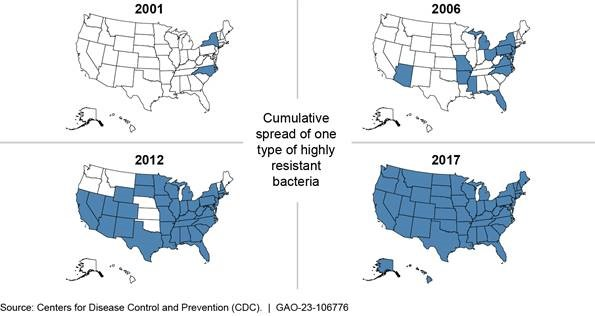
Note: This figure tracks a type of carbapenem-resistant Enterobacteriaceae, which, according to CDC, is a “nightmare bacteria” resistant to nearly all available antibiotics.
Federal agencies have taken steps to advance the development and use of tests to diagnose antibiotic-resistant bacterial infections, but these efforts have limitations. According to experts and agency officials, more studies are needed to help promote the use of these tests. Without information to guide test usage, clinicians may be unable to select appropriate treatments for their patients.
Experts warn that the current pipeline of antibiotics in development is insufficient to meet the threat of resistance. Several challenges impede the development of new treatments for resistant infections, notably inadequate return on investment for drug companies. GAO found in March 2020 that HHS had not developed a strategy to further incentivize development of new treatments, and that HHS may also need to request authority and appropriations to create and implement certain types of incentives.
HHS has begun taking steps to implement six of GAO's eight March 2020 recommendations. For example, it has started the process to draft a strategy to incentivize development of new treatments. However, more work remains. HHS still must take additional actions or finalize efforts to address the challenges GAO identified regarding surveillance, testing, and treatments.
Why GAO Did This Study
Concerns about rising rates of antibiotic resistance prompted the federal government to create the National Action Plan for Combating Antibiotic-Resistant Bacteria in 2015 and again in 2020. The plan called for federal agencies to strengthen surveillance, advance the development of diagnostic tests and new antibiotics, and slow the emergence of resistant bacteria, among other things.
This testimony summarizes GAO's March 2020 report on federal efforts and challenges related to (1) surveillance of antibiotic resistance, (2) diagnostic testing to identify resistant infections, and (3) the development of treatments for resistant infections, among others. This testimony also highlights the recommendations GAO made in that report and includes information on agency progress toward implementing them. For that report, GAO reviewed literature and agency documents; interviewed agency officials and health care industry, drug industry, and other stakeholders; and held a meeting of international and U.S. experts to obtain their views.
Recommendations
GAO made eight recommendations in its March 2020 report. HHS concurred with seven of the recommendations in 2020. It did not concur with the recommendation for a strategy on incentives. However, it has begun taking steps to implement this recommendation and another five. HHS has not taken significant steps on the two remaining recommendations. GAO will continue to monitor HHS's progress.
