National Institutes of Health: Better Data Will Improve Understanding of Federal Contributions to Drug Development
Fast Facts
The National Institutes of Health—the largest public funder of biomedical research in the U.S.—invests billions of dollars each year to help develop new drugs or new uses for existing drugs. But the extent of NIH's contributions to drug development isn't well understood or recognized by the public.
One reason may be that recipients of NIH funds aren't always disclosing agency support fully or correctly when applying for patents.
We recommended that NIH clarify that its awardees should name NIH as the funding agency. This will help the public and policymakers better understand how NIH's investments translate into drugs that benefit Americans.

Highlights
What GAO Found
National Institutes of Health (NIH), an agency in the Department of Health and Human Services (HHS), is the largest public funder of biomedical research and development (R&D). In fiscal years 2017 through 2021, NIH obligated $97 billion for basic research, $28 billion for clinical trials and related activities, and $9 billion for biomedical workforce training, as part of its investments in biomedical R&D.
GAO found that, in fiscal years 2019 through 2022, up to 16 to 18 percent of NIH-funded clinical trials were registered late in the public database ClinicalTrials.gov. The HHS Office of Inspector General reported in August 2022 that only about half of NIH-funded clinical trials submitted results on time to the database in calendar years 2019 and 2020 due to insufficient monitoring and enforcement by NIH. NIH generally requires an NIH-funded clinical trial to be registered within 21 days of enrolling the first participant and results to be reported within 1 year of the trial's completion. NIH officials stated the agency has been taking additional actions since October 2021 to address noncompliance with these requirements, including automated checks for noncompliance and the monitoring of noncompliance rates by analyzing ClinicalTrials.gov data. Timely reporting of information about NIH-funded clinical trials provides transparency of NIH's research to advance drug development.
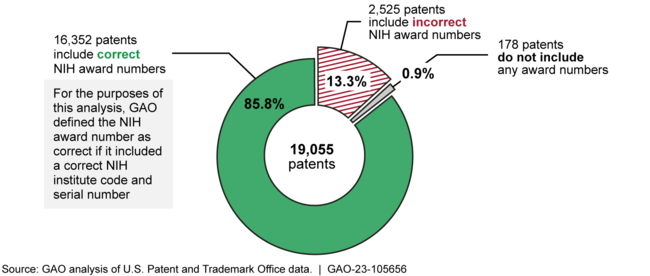
NIH awardees did not consistently disclose NIH support in patents arising from research funded by the agency. GAO found that about 2,700 of 19,055 patents with application dates in calendar years 2012 through 2021 did not fully or correctly disclose NIH support (see figure), as required. NIH does not provide clear guidance that its awardees should name NIH as the funding agency and correctly identify the award number when disclosing NIH support in patents. The disclosure of federal support informs the public and other interested parties of the federal government's involvement. When awardees do not disclose the agency's support correctly, or do not name NIH as the funding agency, these parties cannot link patents to NIH funding and determine the extent of the agency's involvement in developing the patented technologies, including drugs.
Figure: Patents Disclosing Support from the National Institutes of Health (NIH) with Application Dates in Calendar Years 2012 through 2021
Why GAO Did This Study
With a budget of $43 billion in fiscal year 2021, NIH funds multiple R&D activities that contribute to drug development. NIH-funded biomedical R&D generates basic scientific knowledge on biological mechanisms of various diseases, supports clinical trials investigating if drugs are safe and effective, and trains biomedical scientists who go on to work at universities, in government, and industry. Although not all NIH-funded R&D is directly related to drug development, developing drugs and treatments is one of the agency's strategic goals.
GAO was asked to review how NIH-funded biomedical R&D contributes to drug development. This report examines, among other things, (1) NIH funding for basic research, clinical trials, and biomedical workforce training; (2) reporting of information about NIH-funded clinical trials in the public registry ClinicalTrials.gov; and (3) the extent to which NIH support is disclosed in patents arising from research funded by the agency. GAO reviewed relevant laws and agency documents, analyzed clinical trial and patent data, and interviewed NIH officials, grantees, and academic experts.
Recommendations
GAO is making two recommendations to NIH, including that its guidance clarify that awardees should name NIH and include the NIH award number when disclosing the agency's support in patent applications. HHS concurred with the recommendations.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| National Institutes of Health | The Director of NIH should update the Grants Policy Statement and NIH training materials to provide clear guidance that the government interest statement in a patent arising from NIH-funded research should name National Institutes of Health as the federal agency and correctly identify NIH awards using, at the minimum, the institute code and serial number. (Recommendation 1) |
HHS agreed with the recommendation. In response to our recommendation, HHS revised the NIH Grants Policy Statement, Section 8.2.4 Inventions and Patents (Exhibit 8) in April 2024, which now states: "The recipient must include, within the specification of all United States patent applications and any patent issuing thereon covering a Subject Invention, the following statement, 'This invention was made with government support under (grant number, including the two-letter institute code and six-digit serial number, e.g., CA012345) awarded by the National Institutes of Health. The government has certain rights in the invention.'"
|
| National Institutes of Health | The Director of NIH should develop a procedure describing how researchers can access NIH microdata for the purposes of studying and evaluating NIH's contributions to developing new drugs and treatments. (Recommendation 2) |
HHS agreed with the recommendation. In December 2024, NIH launched the Science of Science Scholars Pilot Program to provide researchers with access to the agency's internal administrative data to conduct in-depth analyses of NIH processes, programs, and outcomes of NIH-funded research. Based on the information about pilot program on the agency's website, NIH planned to sponsor two Science of Science scholars for a one-year term in the program's initial year. NIH stated that if successful, NIH would consider expanding the opportunity to more scholars. We consider this pilot program a constructive step toward the implementation of the recommendation. We will update the status of this recommendation when HHS provides information on the results of the pilot's initial one-year term and whether NIH plans to continue making its administrative data available to scholars for research and evaluation purposes.
|
