Institutional Review Boards: Actions Needed to Improve Federal Oversight and Examine Effectiveness
Fast Facts
Institutional Review Boards (IRBs) assess the ethics and safety of research studies involving human subjects, such as behavioral studies or clinical trials for new drugs or medical devices.
Health and Human Services oversees about 2,300 U.S.-based IRBs through routine or for-cause inspections to assess if they are following federal laws when reviewing research. But few IRBs are inspected. For example, one HHS agency aims to do just 3-4 routine inspections each year. Also, HHS agencies haven't examined how many inspections are needed or if inspections could be changed to further reduce risks to human subjects.
Our recommendations address this.

Highlights
What GAO Found
Institutional review boards (IRB) are groups that review ethical and safety considerations for research involving human subjects, such as clinical trials.
General Institutional Review Board (IRB) Process
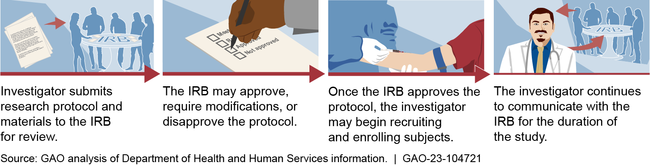
Most IRBs are based at universities, according to Department of Health and Human Services (HHS) data. University-based IRBs were also responsible for reviewing most research involving certain investigational drugs from calendar years 2012 through 2020, according to Food and Drug Administration (FDA) data. Some IRBs are independent, meaning they are not part of institutions that conduct or sponsor research. FDA data show these independent IRBs have reviewed an increasing share of investigational drug research: 25 percent of this research in 2012, and 48 percent in 2021. At the same time, the number of independent IRBs has decreased largely due to consolidation; this is, in part, related to private equity investment in IRBs.
FDA and HHS's Office for Human Research Protections (OHRP) oversee about 2,300 U.S.-based IRBs (operated by about 1,800 separate organizations, which may register and operate one or more IRB) through routine or for-cause inspections. These inspections assess whether IRBs follow federal regulations when reviewing research. FDA and OHRP consider several factors when selecting organizations for inspections, such as the volume of research reviewed. However, GAO found the agencies inspect relatively few IRBs. OHRP officials said they aim to conduct three to four routine inspections annually, while FDA conducted an average of 133 inspections annually between fiscal years 2010 and 2021. Neither agency has conducted a risk-based assessment of their IRB inspection program to help ensure they inspect enough IRBs annually and to optimize their responsibilities in protecting human subjects. Such an approach would be consistent with federal risk management principles.
While the agencies oversee IRBs to determine their adherence to regulations, OHRP and FDA have not assessed to what extent IRB reviews are effective in protecting human subjects. This is because the agencies have not determined the best approaches for doing so. Evaluating effectiveness is challenging in part due to an absence of validated measures and because IRBs are only one part of the framework of stakeholders responsible for protecting human subjects. Convening stakeholders to identify approaches for evaluating IRB effectiveness would be consistent with OHRP and FDA responsibilities and change management practices, and would help provide assurance that IRBs are successful in protecting human subjects.
Why GAO Did This Study
IRBs review research studies involving human subjects to ensure that risks to subjects are minimized and participants have sufficient information to consent to participate. In the past, IRBs were based at research institutions, such as academic centers. Over time, independent IRBs have played a more prominent role in reviewing research on human subjects. Some policymakers and others have raised questions about the increased use of independent IRBs and the effects on protecting human subjects.
GAO was asked to examine independent IRBs, processes used to protect human subjects, and standards of IRB quality, among other things. This report describes the composition of the IRB market and examines OHRP and FDA oversight of IRBs, among other objectives.
GAO reviewed federal laws and regulations and articles published between 2010 and June 2021; analyzed IRB registration, drug application, and inspection data; and interviewed FDA and OHRP officials, experts and stakeholders, and 11 IRBs selected for variation in type, size, and other factors.
Recommendations
GAO is making four recommendations, including that HHS and FDA conduct annual risk assessments to determine if the agencies are routinely inspecting an adequate number of IRBs and to optimize the use of inspections in the oversight of IRBs and protection of research participants, and examine and implement approaches for measuring IRB effectiveness. HHS concurred with the recommendations.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Department of Health and Human Services | The Assistant Secretary for Health should ensure that OHRP takes steps to ensure the accuracy of protocol data collected in OHRP's IRB registry. This could include updating instructions to IRBs and examining data accuracy for a sample of IRBs. (Recommendation 1.) |
In August 2023, HHS reported steps OHRP was taking steps to address this recommendation. HHS noted OHRP was working to modify its IRB registration system to prompt IRBs applying for, renewing, or updating their OHRP registration to provide accurate information about the number of protocols they review. Additionally, HHS reported OHRP planned to evaluate its IRB registration instructions as part of the next Paperwork Reduction Act Information Collection Request renewal; the current IRB registration form is approved through June 30, 2025. In June 2024, OHRP described the importance of reporting accurate IRB registration in a magazine article published earlier in the year. HHS also noted that OHRP is considering ways to obtain information that could help to address this recommendation during its IRB inspections. We will update the status of this recommendation when we receive additional information on the actions OHRP has completed to help ensure the accuracy of the protocol data it collects.
|
| Department of Health and Human Services | The Assistant Secretary for Health should ensure that OHRP conducts an annual risk assessment to determine whether the agency is conducting an adequate number of routine IRB inspections and to optimize the use of IRB inspections in the oversight of IRBs and protection of research participants. (Recommendation 2) |
In August 2023, HHS reported that OHRP was taking steps to address this recommendation. HHS noted OHRP was considering options for conducting the annual risk assessment, and was discussing approaches with FDA and other federal entities that support or conduct human subjects. In April 2024, HHS reiterated the steps it was taking to address this recommendation, noting that identifying which IRBs to inspect will be a complex process that will likely take 2-3 years to successfully implement. We will update the status of this recommendation when we receive additional information regarding actions OHRP has completed to conduct this assessment.
|
| Food and Drug Administration | The Commissioner of the Food and Drug Administration should conduct an annual risk assessment to determine whether the agency is conducting an adequate number of routine IRB inspections and to optimize the use of IRB inspections in the oversight of IRBs and protection of research participants. (Recommendation 3) |
In August 2023, HHS reported that FDA was taking steps to address this recommendation. HHS reported FDA planned to take steps to assess the number of annual IRB inspections it conducts by March 2024, to account for inspections conducted in fiscal year 2023 and 2024. In May 2024, FDA reported conducting this review, and classifying IRBs into types using a similar approach as we described. FDA shared their assessment of IRB inspections conducted in fiscal years and 2023 and 2024. It showed that independent IRBs reviewed approximately half of FDA-regulated clinical research but represented less than 5 percent of IRBs selected for inspection in these fiscal years. FDA also assessed the feasibility of inspecting every IRB once every 5 years and determined it would require more than 300 routine inspections annually, which is 3 times the current number of IRB inspections conducted annually with existing resources. Given these findings, and the disproportionate amount of FDA-regulated research independent IRBs reviewed, FDA said they plan to inspect independent IRBs no less than once every 5 years and will not follow a set timeline for routine inspections of non-independent IRBs. FDA will continue to use its risk-based approach to determine the number of surveillance inspections to conduct of non-independent IRBs. Regarding optimizing the use of IRB inspections, FDA said the agency is exploring other ways to augment IRBs inspections. For example, FDA described beginning a new pilot in fiscal year 2023 to use remote regulatory assessments of IRBs to determine if that approach is a feasible alternative to on-site inspections. Additionally, FDA reported beginning quarterly meetings with OHRP to collaborate on ways to optimize regulatory oversight of IRBs and to decrease duplication in IRB oversight between the two agencies. We will update the status of this recommendation when we receive additional information regarding actions FDA has completed to address this recommendation.
|
| Department of Health and Human Services | The Secretary of Health and Human Services should ensure that OHRP and FDA convene stakeholders to examine approaches for measuring IRB effectiveness in protecting human subjects, and implement the approaches as appropriate. These could include effectiveness measures; peer audits of IRB meetings and decisions; mock protocols; surveys of IRB members, investigators, and human research participants; or other approaches. (Recommendation 4) |
In August 2023, HHS reported that OHRP and FDA had begun identifying and convening stakeholders to examine approaches for measuring IRB effectiveness in protecting human subjects. For example, HHS asked the HHS Secretary's Advisory Committee on Human Research Protections (SACHRP) to consider such approaches; in response, SACHRP issued its recommendations on October 19, 2023. As of June 2024, HHS reported several additional steps taken to help address this recommendation. For example, OHRP and FDA formed a workgroup in October 2023 to focus on this recommendation. According to HHS, OHRP, in collaboration with this group, also solicited feedback on the SACHRP recommendations from the 21 other federal agencies and departments that abide by the Common Rule. Additionally, OHRP and FDA reported collecting feedback on IRB effectiveness and SACHRP recommendations in several stakeholder meetings, and meeting with other experts about potential approaches for examining effectiveness. These are promising steps. We will continue to update the status of this recommendation when we receive additional information on actions OHRP or FDA have completed to examine approaches for measuring IRB effectiveness, and in taking any steps to implement such approaches.
|
