Clinical Labs: Studies Suggest Biopsy Specimen Misidentification and Contamination Errors Are Infrequent
Fast Facts
Healthcare providers use biopsies—removing and examining cells or tissue—to diagnose diseases like cancer. Labs that examine biopsies must ensure that specimens aren't misidentified or contaminated so patients can be properly diagnosed and treated.
Stakeholders estimate that these errors are infrequent. In our review of studies, error rate estimates were generally around 1%, with the highest estimate at 2.3%. These errors could result, for example, from a lab tech mixing up specimens when manually cataloging them.
Technology—like a barcode system to track specimens—and more effective collection and handling procedures may decrease errors.

Highlights
What GAO Found
Health care providers use biopsies—the removal and examination of cells or tissue—to diagnose diseases like cancer. Biopsy specimen source errors include the misidentification or contamination of one patient's biopsy with another.
Centers for Medicare & Medicaid Services (CMS) officials and stakeholders with direct knowledge about specimen source errors told GAO that such errors are infrequent. Representatives from one accreditation organization said they only cited two such errors in the last 2 years. GAO identified six studies that estimated the prevalence of specimen source errors, though these studies cannot be generalized. The highest estimated rate of specimen source errors was 2.3 percent. Studies GAO reviewed attributed specimen source errors to a variety of causes that may occur at different points in the biopsy process. For example, a lab technician may mix up specimens when manually cataloging them upon their arrival to the lab. Integrating technology—like a printed barcode system that allows for specimens to be easily identified and tracked throughout the process—and effective specimen collection and handling procedures may decrease the risk of specimen source errors, according to the literature and stakeholders.
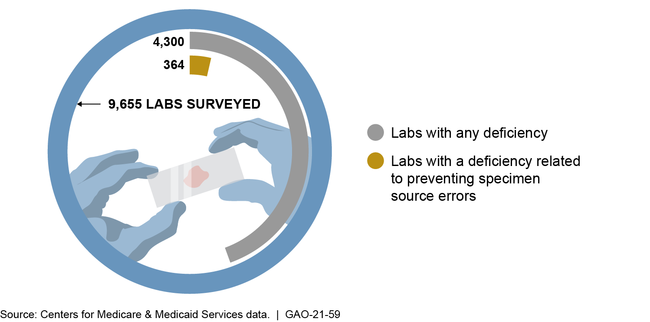
CMS regulations require labs to establish procedures related to preventing specimen source errors. CMS ensures lab compliance with these regulations through biennial inspections conducted either by surveyors acting on behalf of CMS or by lab accreditation organizations. Surveyors also review a sample of labs inspected by each accreditation organization to ensure inspection quality or in response to a complaint. CMS data show that in 2018, 3.8 percent (364 out of 9,655) of labs inspected by surveyors were cited as deficient in at least one of the regulations relevant to specimen source errors. These types of deficiencies were not among the agency's list of the top 10 lab-related deficiencies reported in October 2018.
Lab Inspection Findings in Calendar Year 2018
Why GAO Did This Study
In 2017, over 1.7 million new cases of cancer were reported in the United States. For patients to receive proper diagnosis and treatment, labs must ensure that biopsy specimens are not misidentified or contaminated. Under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), clinical labs that perform tests to diagnose disease must meet requirements. CMS has issued regulations to implement CLIA and is responsible for overseeing and certifying lab compliance with these regulations.
This report describes (1) what is known about the rates and causes of specimen source errors and potential solutions to address them, and (2) CMS's efforts to prevent specimen source errors.
GAO examined peer-reviewed literature published from January 2010 to April 2020 on the rates, causes, and potential solutions of specimen source errors. GAO also reviewed CMS regulations and guidance relevant to preventing specimen source errors, as well as data regarding the number of labs found deficient in meeting those requirements in 2018, the most recent complete data at the time of GAO's review. Finally, GAO interviewed or obtained written responses from CMS officials and representatives from various stakeholder groups, including medical provider organizations, state survey agencies, and lab accreditation organizations. GAO received technical comments on a draft of this report from the Department of Health and Human Services and incorporated them as appropriate.
For more information, contact Jessica Farb at (202) 512-7114 or farbj@gao.gov.
