Federal Research: NIH Should Take Further Action to Address Foreign Influence
Fast Facts
The National Institutes of Health is a major source of research funding for U.S. universities. It has raised concerns over the fruits of this funding going abroad and undisclosed conflicts of interest. For example, in 2020, investigators found a U.S. university researcher involved in a Chinese-government talent recruitment program failed to disclose hundreds of thousands of dollars in foreign income.
We testified that, among other things, NIH:
Does not address non-financial conflicts such as foreign affiliations in its policy
Has mechanisms to ensure that conflict disclosure requirements are met
Helped start 6 criminal investigations

Highlights
What GAO Found
U.S. research may be subject to undue foreign influence in cases where a researcher has a foreign conflict of interest. Federal grant-making agencies, such as the National Institutes of Health (NIH), can address this threat by implementing conflict of interest policies and requiring the disclosure of information that may indicate potential conflicts. GAO found that NIH's policy focuses on financial conflicts of interest but does not specifically address or define non-financial interests, which may include multiple professional appointments. In the absence of agency-wide policies and definitions on non-financial interests, universities that receive federal grant funding may lack sufficient guidance to identify and manage conflicts appropriately, potentially increasing the risk of undue foreign influence. In its report, GAO noted that NIH also requires researchers to disclose information—such as foreign support for their research—as part of grant proposals, and that such information could be used to determine if certain conflicts exist.
National Institutes of Health Disclosure Requirements for Grantees as of December 2020
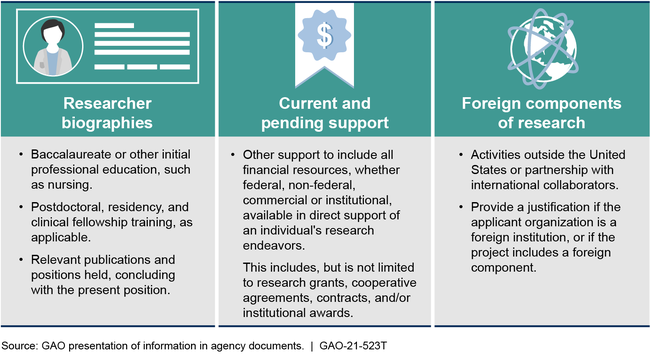
NIH relies on universities to monitor financial conflicts of interest, and the agency collects information, such as foreign collaborations, that could be used to identify non-financial conflicts. NIH has taken action in cases where it identified researchers who failed to disclose financial or non-financial information. Such actions included referring cases to the Department of Justice for criminal investigation. Additionally, NIH has written procedures for addressing allegations of failures to disclose required information.
In interviews, stakeholders identified opportunities to improve agency responses to prevent undue foreign influence in federally funded research. For example, agencies could harmonize grant application requirements and better communicate identified risks. NIH has taken steps to address the issue of foreign influence in the areas stakeholders identified.
Why GAO Did This Study
The federal government reported expending about $44.5 billion on university science and engineering research in fiscal year 2019. The Department of Health and Human Services funds over half of all such federal expenditures, and NIH accounts for almost all of this funding. Safeguarding the U.S. research enterprise from threats of foreign influence is of critical importance. Recent reports by GAO and others have noted challenges faced by the research community to combat undue foreign influence, while maintaining an open research environment.
This testimony discusses (1) NIH's conflict of interest policy and disclosure requirements that address potential foreign influence, (2) NIH's mechanisms to monitor and enforce its policy and requirements, and (3) the steps NIH has taken to address concerns about foreign influence in federally funded research identified by stakeholders. It is based on a report that GAO issued in December 2020 (GAO-21-130).
Recommendations
In its December 2020 report, GAO recommended that NIH define and address non-financial conflicts of interest in its policy. NIH concurred with our recommendation and has recently updated its grant application forms and instructions to require that applicants more fully disclose non-financial interests, including foreign activities and resources. However, NIH has not yet updated its conflict of interest policy.
