COVID-19: Federal Efforts Could Be Strengthened by Timely and Concerted Actions
Fast Facts
This report updates our oversight of federal actions to support public health, individuals, and the economy during the COVID-19 pandemic. Findings include:
There have been shortages of personal protective equipment and testing supplies because very few of them are made in the U.S. and global demand for them is high
HHS may be able to collect more complete data on COVID-19 cases, hospitalizations, and deaths among racial and ethnic minority groups
The Department of the Treasury and the IRS don't know how many eligible people have yet to receive an economic impact payment
We made 16 recommendations to address these and other issues.
COVID-19 experimental vaccine
Highlights
What GAO Found
In the government’s ongoing response to the COVID-19 pandemic, the Congress and the administration have taken action on multiple fronts to address challenges that have contributed to catastrophic loss of life and profound economic disruption. These actions have helped direct much-needed federal assistance to support many aspects of public life, including local public health systems and private-sector businesses.
However, the nation faces continued public health risks and economic difficulties for the foreseeable future. Among other challenges, the public health system, already strained from months of responding to COVID-19 cases, will face the additional task of managing the upcoming flu season. At the same time, many of the federal, state, and local agencies responsible for responding to the ongoing public health emergency are called on to prepare for and respond to the current hurricane season. Timely and concerted federal leadership will be required in responding to these and other challenges.
GAO has identified lessons learned and issues in need of continued attention by the Congress and the administration, including the need to collect reliable data that can drive decision-making; to establish mechanisms for accountability and transparency; and to protect against ongoing cyber threats to patient information, intellectual property, public health data, and intelligence. Attention to these issues can help to make federal efforts as effective as possible.
GAO has also identified a number of opportunities to help the federal government prepare for the months ahead while improving the ongoing federal response:
Medical Supply Chain
The Department of Health and Human Services (HHS) and the Federal Emergency Management Agency (FEMA), with support from the Department of Defense (DOD), have taken numerous, significant efforts to mitigate supply shortages and expand the medical supply chain. For example, the agencies have coordinated to deliver supplies directly to nursing homes and used Defense Production Act authorities to increase the domestic production of supplies.
However, shortages of certain types of personal protective equipment and testing supplies remain due to a supply chain with limited domestic production and high global demand. The Food and Drug Administration (FDA) and FEMA have both identified shortages, and officials from seven of the eight states GAO interviewed in July and August 2020 identified previous or ongoing shortages of testing supplies, including swabs, reagents, tubes, pipettes, and transport media. Testing supply shortages have contributed to delays in turnaround times for testing results. Delays in processing test results have multiple serious consequences, including delays in isolating those who test positive and tracing their contacts in a timely manner, which can in turn exacerbate outbreaks by allowing the virus to spread undetected. In addition, states and other nonfederal entities have experienced challenges tracking supply requests made through the federal government and planning for future needs. GAO is making the following recommendations:
- HHS, in coordination with FEMA, should immediately document roles and responsibilities for supply chain management functions transitioning to HHS, including continued support from other federal partners, to ensure sufficient resources exist to sustain and make the necessary progress in stabilizing the supply chain.
- HHS, in coordination with FEMA, should further develop and communicate to stakeholders plans outlining specific actions the federal government will take to help mitigate supply chain shortages for the remainder of the pandemic.
- HHS and FEMA—working with relevant stakeholders—should devise interim solutions, such as systems and guidance and dissemination of best practices, to help states enhance their ability to track the status of supply requests and plan for supply needs for the remainder of the COVID-19 pandemic response.
HHS and the Department of Homeland Security (DHS) objected to GAO’s initial draft recommendations. GAO made revisions based on their comments. GAO maintains that implementation of its modified recommendations is both warranted and prudent. These actions could contribute to ensuring a more effective response by helping to mitigate challenges with the stability of the medical supply chain and the ability of nonfederal partners to track, plan, and budget for ongoing medical supply needs.
Vaccines and Therapeutics
Multiple federal agencies continue to support the development and manufacturing of vaccines and therapeutics to prevent and treat COVID-19. These efforts are aimed at accelerating the traditional timeline to create a vaccine (see figure).
Traditional Timeline for Development and Creation of a Vaccine
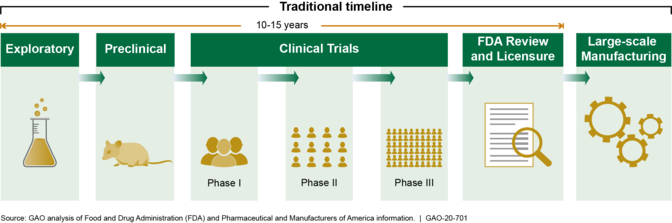
As these efforts proceed, clarity on the federal government’s plans for distributing and administering vaccine, as well as timely, clear, and consistent communication to stakeholders and the public about those plans, is essential. DOD is supporting HHS in developing plans for nationwide distribution and administration of a vaccine. In September 2020, HHS indicated that it will soon send a report to Congress outlining a distribution plan, but did not provide a specific date for doing so. GAO recommends that HHS, with support from DOD, establish a time frame for documenting and sharing a national plan for distributing and administering COVID-19 vaccine, and in developing such a plan ensure that it is consistent with best practices for project planning and scheduling and outlines an approach for how efforts will be coordinated across federal agencies and nonfederal entities. DOD partially concurred with the recommendation, clarifying that it is supporting HHS in developing plans for nationwide distribution and administration of vaccine. HHS neither agreed nor disagreed with the recommendation, but noted factors that complicate the publication of a plan. GAO maintains that a time frame is necessary so all relevant stakeholders will be best positioned to begin their planning.On September 16, 2020, HHS and DOD released two documents outlining a strategy for any COVID-19 vaccine. GAO will evaluate these documents and report on them in future work.GAO will also continue to conduct related work, including examining federal efforts to accelerate the development and manufacturing of COVID-19 vaccines and therapeutics.
COVID-19 Data
Data collected by the Centers for Disease Control and Prevention (CDC) suggest a disproportionate burden of COVID-19 cases, hospitalizations, and deaths exists among racial and ethnic minority groups, but GAO identified gaps in these data. To help address these gaps, on July 22, 2020, CDC released a COVID-19 Response Health Equity Strategy. However, the strategy does not assess whether having the authority to require states and jurisdictions to report race and ethnicity information is necessary to ensure CDC can collect such data. CDC’s strategy also does not specify how it will involve key stakeholders, such as health care providers, laboratories, and state and jurisdictional health departments. GAO recommends that CDC (1) determine whether having the authority to require the reporting of race and ethnicity information for cases, hospitalizations, and deaths is necessary for ensuring more complete data, and if so, seek such authority from Congress; (2) involve key stakeholders to help ensure the complete and consistent collection of demographic data; and (3) take steps to help ensure its ability to comprehensively assess the long-term health outcomes of persons with COVID-19, including by race and ethnicity. HHS agreed with the recommendations.
In addition, HHS’s data on COVID-19 in nursing homes do not capture the early months of the pandemic. HHS’s Centers for Medicare & Medicaid Services (CMS) began requiring nursing homes to report COVID-19 data to CDC by May 17, 2020, starting with information as of May 8, 2020, but made reporting prior to May 8, 2020 optional. By not requiring nursing homes to submit data from the first 4 months of 2020, HHS is limiting the usefulness of the data in helping to understand the effects of COVID-19 in nursing homes. GAO recommends that HHS, in consultation with CMS and CDC, develop a strategy to capture more complete data on COVID-19 cases and deaths in nursing homes retroactively back to January 1, 2020. HHS partially agreed with this recommendation by noting the value of having complete data, but expressed concern about the burden of collecting it. GAO maintains the importance of collecting these data to inform the government’s continued response and recovery, and HHS could ease the burden by incorporating data previously reported to CDC or to state or local public health offices.
Economic Impact Payments
The Department of the Treasury’s (Treasury) Internal Revenue Service (IRS) has issued economic impact payments (EIP) to all eligible individuals for whom IRS has the necessary information to do so; however, not everyone eligible was able to be initially identified. To help ensure all eligible recipients received their payments in a more timely manner, IRS took several actions to address challenges GAO reported on in June, including a policy change—reopening the Non-Filers tool registration period for federal benefit recipients and extending it through September 30—that should allow some eligible recipients to receive supplemental payments for qualifying children sooner than expected. However, Treasury and IRS lack updated information on how many eligible recipients have yet to receive these funds. The lack of such information could hinder outreach efforts and place potentially millions of individuals at risk of missing their payment. GAO recommends that Treasury, in coordination with IRS, (1) update and refine the estimate of eligible recipients who have yet to file for an EIP to help target outreach and communications efforts and (2) make estimates of eligible recipients who have yet to file for an EIP, and other relevant information, available to outreach partners to raise awareness about how and when to file for EIP. Treasury and IRS neither agreed nor disagreed with the recommendations and described actions they are taking in concert with the recommendations to notify around 9 million individuals who may be eligible for an EIP.
Coronavirus Relief Fund
The Coronavirus Relief Fund (CRF) is the largest program established in the four COVID-19 relief laws that provides aid to states, the District of Columbia, localities, tribal governments, and U.S. territories. Audits of entities that receive federal funds, including CRF payments, are critical to the federal government’s ability to help safeguard those funds. Auditors that conduct single audits follow guidance in the Single Audit Act’s Compliance Supplement, which the Office of Management and Budget (OMB) updates and issues annually in coordination with federal agencies. OMB issued the 2020 Compliance Supplement in August 2020, but the Compliance Supplement specified that OMB is still working with federal agencies to identify the needs for additional guidance for auditing new COVID-19-related programs, including the CRF payments, as well as existing programs with compliance requirement changes. According to OMB, an addendum on COVID-19-related programs, including the CRF payments, will be issued in the fall of 2020. Further delays in issuing this guidance could adversely affect auditors’ ability to issue consistent and timely reports. GAO recommends that OMB, in consultation with Treasury, issue the addendum to the 2020 Compliance Supplement as soon as possible to provide the necessary audit guidance, as many single audit efforts are underway. OMB neither agreed nor disagreed with the recommendation.
Guidance for K-12 Schools
State and local school district officials tasked with reassessing their operating status and ensuring their school buildings are safe are generally relying on guidance and recommendations from federal, state, and local public health and education officials. However, portions of CDC’s guidance on reopening K-12 schools are inconsistent, and some federal guidance appears misaligned with CDC’s risk-based approach on school operating status. Based on GAO’s review, Education has updated the information and CDC has begun to do so. GAO recommends that CDC ensure that, as it makes updates to its guidance related to schools’ operating status, the guidance is cogent, clear, and internally consistent. HHS agreed with the recommendation.
Tracking Contract Obligations
Federal agencies are tracking contract actions and associated obligations in response to COVID-19 using a National Interest Action (NIA) code in the Federal Procurement Data System-Next Generation. The COVID-19 NIA code was established in March 2020 and was recently extended until March 31, 2021, while a draft of this report recommending that DHS and DOD extend the code beyond September 30, 2020, was with the agencies for comment. GAO has identified inconsistencies in establishing and closing these codes following previous emergencies, and has continued concerns with the criteria that DHS and DOD rely on to determine whether to extend or close a code and whether the code meets long-term needs. GAO recommends that DHS and DOD make updates to the 2019 NIA Code Memorandum of Agreement so as to enhance visibility for federal agencies, the public, and Congress on contract actions and associated obligations related to disaster events, and to ensure the criteria for extending or closing the NIA code reflect government-wide needs for tracking contract actions in longer-term emergencies, such as a pandemic. DHS and DOD did not agree, but GAO maintains implementation of its recommendation is essential.
Address Cybersecurity Weaknesses
Since March 2020, malicious cyber actors have exploited COVID-19 to target organizations that make up the health care and public health critical infrastructure sector, including government entities, such as HHS. GAO has identified numerous cybersecurity weaknesses at multiple HHS component agencies, including CMS, CDC, and FDA, over the last 6 years, such as weaknesses in key safeguards to limit, prevent, and detect inappropriate access to computer resources. Additionally, GAO’s March 2019 high-risk update identified cybersecurity and safeguarding the systems supporting the nation’s critical infrastructure, such as health care, as high-risk areas. As of July 2020, CMS, FDA, and CDC had made significant progress by implementing 350 (about 81 percent) of the 434 recommendations GAO issued in previous reports to address these weaknesses. Based on the imminent cybersecurity threats, GAO recommends that HHS expedite implementation of GAO’s prior recommendations regarding cybersecurity weaknesses at its component agencies. HHS agreed with the recommendation.
Why GAO Did This Study
As of September 10, 2020, the U.S. had over 6.3 million cumulative reported cases of COVID-19 and over 177,000 reported deaths, according to federal agencies. The country also continues to experience serious economic repercussions and turmoil.
Four relief laws, including the CARES Act, were enacted as of September 2020 to provide appropriations to address the public health and economic threats posed by COVID-19. As of July 31, 2020, the federal government had obligated a total of $1.6 trillion and expended $1.5 trillion of the COVID-19 relief funds as reported by federal agencies on USAspending.gov.
The CARES Act includes a provision for GAO to report bimonthly on its ongoing monitoring and oversight efforts related to the COVID-19 pandemic. This third report examines key actions the federal government has taken to address the COVID-19 pandemic and evolving lessons learned relevant to the nation’s response to pandemics.
GAO reviewed data, documents, and guidance from federal agencies about their activities and interviewed federal and state officials, as well as industry representatives.
Recommendations
GAO is making 16 new recommendations for agencies that are detailed in this Highlights and in the report.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Department of Health and Human Services |
Priority Rec.
The Secretary of Health and Human Services in coordination with the Administrator of the Federal Emergency Management Agency—who head agencies leading the COVID-19 response through the Unified Coordination Group—should immediately document roles and responsibilities for supply chain management functions transitioning to the Department of Health and Human Services, including continued support from other federal partners, to ensure sufficient resources exist to sustain and make the necessary progress in stabilizing the supply chain, and address emergent supply issues for the duration of the COVID-19 pandemic. (Recommendation 1) |
While the Department of Health and Human Services (HHS) disagreed with our recommendation, the Department, in conjunction with other federal partners, has taken several steps that fulfill the intent of this recommendation. For example, in May 2021, HHS and the Department of Defense (DOD) signed a Memorandum of Understanding in effect through September 2023 expressing DOD's commitment to provide HHS continued acquisition assistance until acquisition oversight and support can be provided organically by HHS. Additionally, in September 2021, the administration publicly released the National Strategy for a Resilient Public Health Supply Chain (National Strategy), developed in collaboration among several departments and signed by the Secretaries Health and Human Services, Defense, Homeland Security, and Veteran's Affairs. HHS is the lead agency for implementing the National Strategy, which includes as a goal transforming the federal government's ability to monitor and manage the public health supply chain through stockpiles, visibility, and engagement with partners within state, local, tribal, and territorial governments and the private-sector. Further, HHS, in conjunction with interagency partners, developed additional plans to help implement the National Strategy. Among other elements, these plans were to provide information on organizational roles and responsibilities and resource needs. As we reported in April 2022 (GAO-22-105397), based on our review of some of the draft implementation plans, the National Strategy and implementation plans provide reasonable assurance that an effective national supply strategy is being developed if they continue as planned. Additionally, HHS officials noted that they expect to make progress updates on the implementation of the National Strategy public. Finally, in October 2022, the White House released an updated National Biodefense Strategy and Implementation Plan, which acknowledges the work needed to improve supply chain resiliency and identifies lead and supporting federal departments responsible for developing a resilient and scalable supply of personal protective equipment. These activities collectively address the intent of our recommendation as the pandemic continues.
|
| Department of Health and Human Services |
Priority Rec.
The Secretary of Health and Human Services in coordination with the Administrator of the Federal Emergency Management Agency—who head agencies leading the COVID-19 response through the Unified Coordination Group—should further develop and communicate to stakeholders plans outlining specific actions the federal government will take to help mitigate remaining medical supply gaps necessary to respond to the remainder of the pandemic, including through the use of Defense Production Act authorities. (Recommendation 2) |
While the Department of Health and Human Services (HHS) disagreed with our recommendation, the Department, in conjunction with other federal partners, has taken several steps to help mitigate supply gaps that fulfill the intent of this recommendation. For example, in September 2020, HHS formed a new Innovation and Industrial Base Expansion Program Office. As we reported in April 2022 (GAO-22-105397), one of the main goals of this office is to expand, secure, and build resilience across the U.S. public health industrial base to address vulnerabilities highlighted during the pandemic, such as critical deficiencies in certain medical supplies. One of the initial tasks of this office was managing $10 billion to support the use of the Defense Production Act for the purchase, production, and distribution of medical supplies related to addressing the COVID-19 pandemic. Additionally, in September 2021, the administration publicly released the National Strategy for a Resilient Public Health Supply Chain (National Strategy), developed in collaboration among several departments and signed by the Secretaries Health and Human Services, Defense, Homeland Security, and Veteran's Affairs. HHS is the lead agency for implementing the National Strategy, which includes as a goal transforming the federal government's ability to monitor and manage the public health supply chain through stockpiles, visibility, and engagement with partners within state, local, tribal, and territorial governments and the private-sector. Additionally, HHS officials noted that they expect to make progress updates on the implementation of the National Strategy public. Finally, HHS, through the Strategic National Stockpile (SNS), has stockpiled significant amounts of personal protective equipment in an effort to mitigate supply gaps and combat future surges. In early 2022, the SNS released 400 million N-95 masks to the public in an effort to provide free, higher quality masks during a COVID-19 surge. As noted on the SNS website, after this deployment the SNS had 350 million N95 masks available for healthcare workers. These activities collectively address the intent of our recommendation.
|
| Department of Health and Human Services | The Secretary of Health and Human Services—who heads one of the agencies leading the COVID-19 response through the Unified Coordination Group—consistent with their roles and responsibilities, should work with relevant federal, state, territorial, and tribal stakeholders to devise interim solutions, such as systems and guidance and dissemination of best practices, to help states enhance their ability to track the status of supply requests and plan for supply needs for the remainder of the COVID-19 pandemic response. (Recommendation 3) |
In April 2023, HHS noted that the Department now agrees with this recommendation, a change to its prior position. HHS also provided additional information to support the steps it took during the COVID-19 pandemic to communicate with state, territorial, and tribal stakeholders to assist them in tracking supply requests and planning supply needs. Specifically, HHS noted that in March 2023, ASPR updated guidance on its website that outlines how state, local, territorial, and tribal stakeholders can request and receive SNS assets. HHS also provided additional documentation that highlighted mechanisms of communication with jurisdictions including to support COVID-19 therapeutics ordering, to support consistent communications between ASPR regional offices and state or other partners, and monthly calls with states to discuss medical countermeasure distribution and dispensing. For example, ASPR initially hosted twice a week stakeholder calls-which shifted to once a week in May 2022-for health officials and healthcare and hospital associations to provide the latest information on COVID-19 therapeutics including changes to ordering and distribution processes. In 2023, these calls have transitioned to every other week. Additionally, HHS provided us documentation of state specific reports or summaries they shared with state and other officials during the COVID-19 pandemic. For example, in early 2021 HHS developed and disseminated state profile reports that provided data on the percentage of hospitals in each state with PPE supply shortages, among other things. State profile reports ceased in February 2023. These efforts fulfill the intent of this recommendation to provide jurisdictions with enhanced ability to track supply requests and plan for supply needs for the remainder of the pandemic.
|
| Federal Emergency Management Agency |
Priority Rec.
The Administrator of the Federal Emergency Management Agency—who heads one of the agencies leading the COVID-19 response through the Unified Coordination Group—consistent with their roles and responsibilities, should work with relevant federal, state, territorial, and tribal stakeholders to devise interim solutions, such as systems and guidance and dissemination of best practices, to help states enhance their ability to track the status of supply requests and plan for supply needs for the remainder of the COVID-19 pandemic response. (Recommendation 4) |
In September 2020, the Department of Homeland Security (DHS) disagreed with this recommendation, noting, among other things, work that FEMA had already done to manage the medical supply chain and increase supply availability. Although DHS disagreed with our recommendation, it took several actions in 2021 and 2022. First, in 2021, FEMA developed and released an updated distribution management plan guide that, according to FEMA, provides actionable guidance for state, local, tribal, and territorial agencies, among others, to effectively and efficiently distribute critical resources to disaster survivors in the community. Second, FEMA published and put online a logistics technical assistance program, which assists in the development, readiness, and enhancement of logistics planning and operational capabilities for state, local, tribal and territorial agencies. Third, FEMA provided information about listening sessions conducted at the regional level. In December 2022, FEMA provided a draft copy of the National Framework for Allocation of Constrained Public Health Resources. The framework establishes a methodology by which federal departments, agencies, and interagency groups may create processes to address constrained federal public health and medical resources when a public health incident arises or if public health and medical resources are constrained. In November 2023, FEMA reported that HHS is the executive agent for all actions associated with the National Strategy for a Resilient Public Health Supply Chain; they are building an Administration for Strategic Preparedness and Response Supply Chain Site that will house the framework and all other documents finalized as part of the combined effort. As a result, no additional actions are warranted by DHS, and we consider this recommendation closed and implemented.
|
| Department of Health and Human Services |
Priority Rec.
The Secretary of Health and Human Services, with support from the Secretary of Defense, should establish a time frame for documenting and sharing a national plan for distributing and administering COVID-19 vaccine, and in developing such a plan ensure that it is consistent with best practices for project planning and scheduling and outlines an approach for how efforts will be coordinated across federal agencies and nonfederal entities. (Recommendation 5) |
Since September 2020, the Centers for Disease Control and Prevention (CDC) and the White House issued vaccine planning documents, including a National Strategy for the COVID-19 Response and Pandemic Preparedness that broadly outline various programs for vaccine distribution. These documents contained general information on federally supported vaccine distribution activities, but did not provide details related to how the federal government was coordinating its efforts or information on the specific roles of the federal agencies and non-federal entities. We closed this recommendation in April 2022 because the time frame for its implementation had passed, due to widespread distribution and administration of COVID-19 vaccines.
|
| Centers for Disease Control and Prevention | As the Centers for Disease Control and Prevention (CDC) implements its COVID-19 Response Health Equity Strategy, the Director of the Centers for Disease Control and Prevention should determine whether having the authority to require states and jurisdictions to report race and ethnicity information for COVID-19 cases, hospitalizations, and deaths is necessary for ensuring more complete data, and if so, seek such authority from Congress. (Recommendation 6) |
CDC agreed with our recommendation. In response, CDC stated that it was reviewing race and ethnicity data completeness across its core surveillance systems and engaging stakeholders from across the agency and in state and local health departments to improve the collection of race and ethnicity data. CDC noted that stakeholders include CDC leadership, key task forces from within CDC's COVID-19 emergency response, and data and surveillance experts in CDC and state health agencies. CDC reported that the information derived from this review would be discussed with the CDC Director and used to assess potential opportunities to enhance the collection of race and ethnicity data, including seeking policy changes or legislative authorities. In August 2021, CDC stated that it's Deputy Director for Public Health Science and Surveillance determined that additional legal authorities to require or better incentivize reporting of public health data may help improve the reporting of race and ethnicity information both for COVID-19 and other diseases. CDC stated this determination will inform the agency's interactions with and technical assistance provided to Congress on legal authority for public health data collection.
|
| Centers for Disease Control and Prevention | As CDC implements its COVID-19 Response Health Equity Strategy, the Director of the Centers for Disease Control and Prevention should involve key stakeholders to help ensure the complete and consistent collection of demographic data. (Recommendation 7) |
CDC agreed with our recommendation. In response, CDC stated that it was reviewing the quality of demographic data, including the completeness of race and ethnicity data, across its core surveillance systems and engaging stakeholders from across the agency and in state and local health departments on the issue. CDC noted that stakeholders include CDC leadership, key task forces from within CDC's COVID-19 emergency response, and data and surveillance experts in CDC and state health agencies. In May 2021, CDC reported that it had conducted listening sessions with community health workers who serve communities of color and rural populations to seek input on the importance of collecting race and ethnicity data. CDC stated that the information collected will inform the development of appropriate and tailored messages that can be used by community health workers to educate communities about the importance of providing race and ethnicity data when receiving health services, overcome hesitance in sharing this information, and describe how this information is used to promote community health. In addition, CDC stated that it is working with public health partners to automate the generation and transmission to CDC of COVID-19 case reports that contain demographic information, including race and ethnicity. In August 2021, CDC stated that monitoring and assessing the completeness of race and ethnicity data in its COVID-19 data systems remains a priority and that addressing the completeness of demographic data is a part of larger ongoing Agency Data Modernization Initiatives. For example, the agency collaborated with the Council of State and Territorial Epidemiologists on an assessment to learn about factors affecting the completeness and quality of race and ethnicity data across various systems. CDC stated that findings from this assessment will inform ongoing collaboration with public health organizations, jurisdictions, and other federal partners and be integrated into existing work in this area.
|
| Centers for Disease Control and Prevention |
Priority Rec.
As CDC implements its COVID-19 Response Health Equity Strategy, the Director of the Centers for Disease Control and Prevention should take steps to help ensure CDC's ability to comprehensively assess the long-term health outcomes of persons with COVID-19, including by race and ethnicity. (Recommendation 8) |
CDC agreed with our recommendation. In response, CDC noted in October 2020 that it was convening a team to develop a plan to monitor the long-term health outcomes of persons with COVID-19 by identifying health care surveillance systems that can electronically report health conditions to state and local health departments. In May 2021, CDC reported that it had various efforts underway with external partners to assess long-term health outcomes. For example, CDC was funding a number of prospective studies in partnership with universities to understand the long-term effects of COVID-19, including a study examining the neurological health outcomes of a large cohort of Black and Hispanic or Latino persons who had COVID-19. It reported that its ongoing studies will follow patients for up to two years and provide information on the percentage of people who develop post-COVID-19 conditions and assess risk factors for the development of these conditions. According to CDC, these studies will assess different virus strains and antibody responses and the underlying immune response in people who develop post-COVID conditions. In August 2021, CDC reported that the agency continues to conduct studies to assess long-term health outcomes of persons with COVID-19, including by race and ethnicity. For example, CDC reported that it is partnering with an academic institution to study long-term symptoms in 2000 adults who tested positive for COVID-19 following them for three years to describe whether symptoms improve or worsen over time.
|
| Department of the Treasury | The Secretary of the Treasury, in coordination with the Commissioner of Internal Revenue, should update and refine the estimate of eligible recipients who have yet to file for an economic impact payment to help target outreach and communications efforts. (Recommendation 9) |
Treasury and IRS took actions that were consistent with this recommendation including Treasury revising its estimate of eligible recipients who had not yet filed for an EIP, publishing a file containing, by zip code, the number of children who may be eligible to be claimed for the advance CTC, and analyzing the income tax filing responses of individuals who were mailed the first-round EIP outreach letters in September 2020.
|
| Department of the Treasury | The Secretary of the Treasury, in coordination with the Commissioner of Internal Revenue, should make estimates of eligible recipients who have yet to file for an economic impact payment, and other relevant information, available to outreach partners to raise awareness about how and when to file for economic impact payments. (Recommendation 10) |
Treasury and IRS took actions that were consistent with this recommendation including Treasury revising its estimate of eligible recipients who had not yet filed for an EIP, publishing a file containing, by zip code, the number of children who may be eligible to be claimed for the advance CTC, and analyzing the income tax filing responses of individuals who were mailed the first-round EIP outreach letters in September 2020.
|
| Office of Management and Budget | The Director of the Office of Management and Budget, in consultation with the Department of the Treasury, should issue the addendum to the 2020 Compliance Supplement as soon as possible to provide the necessary audit guidance. (Recommendation 11) |
The Office of Management and Budget (OMB) neither agreed nor disagreed with the recommendation. OMB issued the 2020 Compliance Supplement Addendum on December 22, 2020.
|
| Centers for Disease Control and Prevention | The Director of the Centers for Disease Control and Prevention should ensure that, as it makes updates to its federal guidance related to reassessing schools' operating status, the guidance is cogent, clear, and internally consistent. (Recommendation 12) |
This recommendation is closed as implemented. CDC's guidance for school operating status during COVID-19 is more cogent, clear, and consistent. On February 12, 2021, CDC released revised guidance for returning to in-person learning, as well as mitigation strategies to help prevent and reduce the spread of COVID-19 in school settings. We found the guidance consolidated much of the earlier guidance into one document that clearly displays all five of CDC's mitigation strategies and includes steps school officials should consider when deciding to reopen schools. In addition, we identified increased efforts to synchronize content across CDC's website. We found that CDC had removed some and updated other information and had begun including summaries of changes made to the guidance at the top of some webpages.
|
| Department of Homeland Security | The Secretary of Homeland Security, in coordination with the Secretary of Defense, should (1) revise the criteria in the 2019 National Interest Action code memorandum of agreement to clearly identify steps they will take to obtain input from key federal agencies prior to extending or closing a National Interest Action code, (2) establish timelines for evaluating the need to extend a National Interest Action code, and (3) define what constitutes a consistent decrease in contract actions and routine contract activity to ensure the criteria for extending or closing the National Interest Action code reflect government-wide needs for tracking contract actions in longer term emergencies, such as a pandemic. (Recommendation 13) |
DHS did not agree with our recommendation. However, in March 2021, DHS, in coordination with DOD, issued a revised memorandum of agreement. The revised agreement establishes a process and timelines for communicating and evaluating National Interest Action code extensions by requiring the General Services Administration to notify other federal agencies no less than 7 days before a code is set to expire so that agencies can request an extension, as needed. The revised agreement also more clearly defines what constitutes a consistent decrease in contract actions to ensure that criteria for extending or closing a National Interest Action code are consistently applied.
|
| Department of Defense | The Secretary of Defense, in coordination with the Secretary of Homeland Security, should (1) revise the criteria in the 2019 National Interest Action code memorandum of agreement to clearly identify steps they will take to obtain input from key federal agencies prior to extending or closing a National Interest Action code, (2) establish timelines for evaluating the need to extend a National Interest Action code, and (3) define what constitutes a consistent decrease in contract actions and routine contract activity to ensure the criteria for extending or closing the National Interest Action code reflect government-wide needs for tracking contract actions in longer term emergencies, such as a pandemic. (Recommendation 14) |
The Department of Defense (DOD) did not agree with our recommendation. However, in March 2021 DOD, in coordination with DHS, issued a revised memorandum of agreement. The revised agreement establishes a process and timelines for communicating and evaluating National Interest Action code extensions by requiring the General Services Administration to notify other federal agencies no less than 7 days before a National Interest Action code is set to expire so that agencies can request an extension as needed. The revised agreement also more clearly defines what constitutes a consistent decrease in contract actions to ensure criteria for extending or closing a National Interest Action code are consistently applied.
|
| Department of Health and Human Services |
Priority Rec.
The Secretary of Health and Human Services, in consultation with the Centers for Medicare & Medicaid Services and CDC, should develop a strategy to capture more complete data on confirmed COVID-19 cases and deaths in nursing homes retroactively back to January 1, 2020, and to clarify the extent to which nursing homes have reported data before May 8, 2020. To the extent feasible, this strategy to capture more complete data should incorporate information nursing homes previously reported to CDC or to state or local public health offices. (Recommendation 15) |
HHS partially agreed with this recommendation. However, as of October 2023, this recommendation remains unimplemented. As we enter the fourth year since the beginning of the pandemic, we have determined that retrospectively collecting COVID-19 case and death data prior to May 8, 2020, could prove burdensome for both nursing homes and HHS and detract resources that would be otherwise used to support resident care. Additionally, there may be potential issues with retrospective data reporting during that period compared to how the data are reported today, given multiple factors, like differences in the availability in testing at that time. HHS expects to sunset most of the COVID-19 reporting requirements by December 31, 2024. Further, HHS noted in previous correspondence that the Agency does not believe that obtaining this data would lead to different or additional actions for emergency response purposes. For these reasons, GAO has decided to close this recommendation as no longer valid.
|
| Department of Health and Human Services | Based on the imminent cybersecurity threats, the Secretary of Health and Human Services should expedite implementation of our prior recommendations regarding cybersecurity weaknesses at its component agencies. (Recommendation 16) |
As of April 2024, HHS closed all 434 prior recommendations that we issued to the Centers for Medicare and Medicaid Services, Food and Drug Administration, and Centers for Drug Control and Prevention to address cybersecurity weaknesses at its component agencies.
|
