Prescription Opioids: Patient Options for Safe and Effective Disposal of Unused Opioids
Fast Facts
An estimated 11.1 million Americans misused a prescription pain reliever, including opioids, in 2017. One of the ways government agencies and others have attempted to address misuse is by facilitating safe disposal of these drugs.
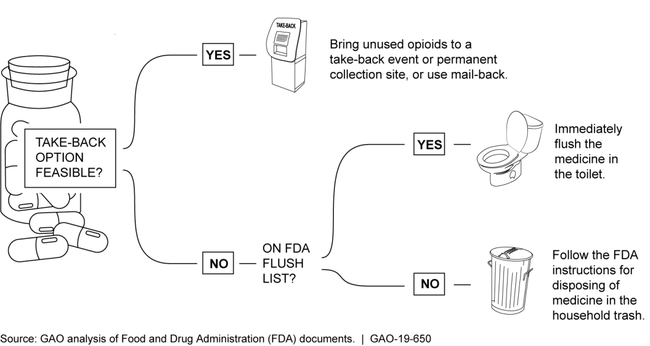
Federal agencies recommend that patients bring unused prescription opioids to registered collection sites or take-back events, or return them via a mail-back program. If these aren’t feasible, the FDA recommends rapid disposal of certain dangerous opioids—such as fentanyl—down a toilet.
Studies show most people are unaware of recommended disposal methods or have not disposed of unused prescription opioids.

Pills and pill bottles scattered on a white surface
Highlights
What GAO Found
The Food and Drug Administration (FDA), Drug Enforcement Administration (DEA), and Environmental Protection Agency (EPA) recommend that patients dispose of unused presciption opioids by bringing them to DEA-registered collection sites or a DEA take-back event, or using mail-back programs. As of April 2019, 70 percent of the U.S. population lived less than 5 miles from permanent collection sites, which are often located at pharmacies. If collection sites, take-back events, or mail-back programs are not feasible, FDA recommends quickly and permanently removing the most dangerous prescription opioids, such as hydrocodone and fentanyl, from the home by flushing them down the toilet. For all other prescription opioids, the agencies recommend disposal in the trash after mixing them with unpalatable substances, such as cat litter. Commercial products to facilitate in-home disposal also exist, and FDA is aware that patients may opt to use these products for disposal in the trash.
FDA Recommendations for Disposal of Unused Prescription Opioids
Available studies suggest that many patients are unaware of federally recommended disposal methods or choose not to dispose of unused prescription opioids. For example, five studies found that between one-quarter and three-quarters of patients stored unused opioids for future use or had misplaced their unused opioids. Further, federal data indicate that 85 percent of intentional misuse occurs with the patient's knowledge—for example, when a patient sells or gives away unused prescription opioids. To educate and motivate patients to dispose of unused opioids, FDA launched a public awareness campaign called “Remove the Risk” in April 2019. Also, FDA and other stakeholders have created educational materials for patients and providers on safe opioid disposal.
Why GAO Did This Study
In 2017, an estimated 11.1 million Americans misused a prescription pain reliever, which included opioids. This misuse contributes to opioid abuse and death, which has quintupled from 1999 to 2017; about 17,000 people died from prescription opioid overdoses in 2017. Government agencies and stakeholders have attempted to address the potential for misuse and abuse by facilitating safe disposal of unused prescription opioids and other drugs.
The SUPPORT for Patients and Communities Act enacted in 2018 included a provision for GAO to review patient disposal of unused opioids, among other things. This report examines (1) federally recommended and other available methods patients may use to dispose of unused prescription opioids, and (2) what is known about patients' use of these methods.
To do this work, GAO examined peer-reviewed, academic literature on outcomes for prescription opioid disposal; reviewed federal agency documentation; interviewed federal agency officials, independent researchers, and stakeholder group representatives—such as those from the American Medical Association; and analyzed DEA data as of April 2019 on permanent drug collection sites. GAO also interviewed representatives of three companies that manufacture commercial in-home disposal products and reviewed publicly available documents about these products.
For more information, contact James Cosgrove at (202) 512-7114 or cosgrovej@gao.gov.
