Medicare: CMS Should Take Actions to Continue Prior Authorization Efforts to Reduce Spending
Fast Facts
In an effort to reduce improper payments and reduce expenses, the Medicare program has experimented with requiring prior authorization. This means beneficiaries need approval before they can receive certain services or items like powered wheelchairs.
We found this approach, which started in 7 states in 2012, reduced spending on these items and services by as much as $1.9 billion. Providers and suppliers reported benefits from the approach, but also had concerns about uncertainties created over what is covered.
Most prior authorization programs are slated to end. We recommended Medicare take steps to continue prior authorization.
Prior Authorization Programs’ Implementation and End Dates
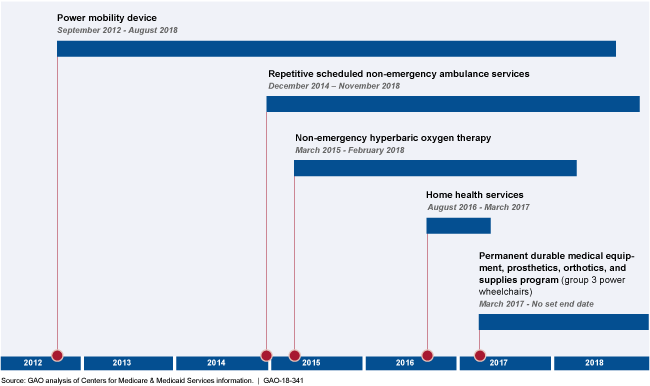
This graphic shows implementation and end dates for each prior authorization program.
Highlights
What GAO Found
Prior authorization is a payment approach used by private insurers that generally requires health care providers and suppliers to first demonstrate compliance with coverage and payment rules before certain items or services are provided to patients, rather than after the items or services have been provided. This approach may be used to reduce expenditures, unnecessary utilization, and improper payments. The Centers for Medicare & Medicaid Services (CMS) has begun using prior authorization in Medicare through a series of fixed-length demonstrations designed to measure their effectiveness, and one permanent program. According to GAO's analyses, expenditures decreased for items and services subject to a demonstration. GAO's analyses of actual expenditures and estimated expenditures in the absence of the demonstrations found that estimated savings from all demonstrations through March 2017 could be as high as about $1.1 to $1.9 billion. While CMS officials said that prior authorization likely played a large role in reducing expenditures, it is difficult to separate the effects of prior authorization from other program integrity efforts. For example, CMS implemented a durable medical equipment competitive bidding program in January 2011, and according to the agency, it resulted in lower expenditures.
Many provider, supplier, and beneficiary group officials GAO spoke with reported benefits of prior authorization, such as reducing unnecessary utilization. However, provider and supplier group officials reported that providers and suppliers experienced some challenges. These include difficulty obtaining the necessary documentation from referring physicians to submit a prior authorization request, although CMS has created templates and other tools to address this concern. In addition, providers and suppliers reported concerns about whether accessories deemed essential to the power wheelchairs under the permanent durable medical equipment, prosthetics, orthotics, and supplies (DMEPOS) program are subject to prior authorization. In practice, Medicare Administrative Contractors (MAC) that administer prior authorization programs review these accessories when making prior authorization determinations, even though they are not technically included in the program and therefore cannot be provisionally affirmed. As a result, providers and suppliers lack assurance about whether Medicare is likely to pay for these accessories. This is contrary to a CMS stated benefit of prior authorization—to provide assurance about whether Medicare is likely to pay for an item or service—and to federal internal control standards, which call for agencies to design control activities that enable an agency to achieve its objectives.
CMS monitors prior authorization through various MAC reports. CMS also reviews MAC accuracy and timeliness in processing prior authorization requests and has contracted for independent evaluations of the demonstrations. Currently, prior authorization demonstrations are scheduled to end in 2018. Despite its interest in using prior authorization for additional items, CMS has not made plans to continue its efforts. Federal internal control standards state that agencies should identify, analyze, and respond to risks related to achieving objectives. CMS risks missed opportunities for achieving its stated goals of reducing costs and realizing program savings by reducing unnecessary utilization and improper payments.
Why GAO Did This Study
CMS required prior authorization as a demonstration in 2012 for certain power mobility devices, such as power wheelchairs, in seven states. Under the prior authorization process, MACs review prior authorization requests and make determinations to approve or deny them based on Medicare coverage and payment rules. Approved requests will be paid as long as all other Medicare payment requirements are met.
GAO was asked to examine CMS's prior authorization programs. GAO examined 1) the changes in expenditures and the potential savings for items and services subject to prior authorization demonstrations, 2) reported benefits and challenges of prior authorization, and 3) CMS's monitoring of the programs and plans for future prior authorization. To do this, GAO examined prior authorization program data, CMS documentation, and federal internal control standards. GAO also interviewed CMS and MAC officials, as well as selected provider, supplier, and beneficiary groups.
Recommendations
GAO recommends that CMS (1) subject accessories essential to the power wheelchairs in the permanent DMEPOS program to prior authorization and (2) take steps, based on results from evaluations, to continue prior authorization. The Department of Health and Human Services neither agreed nor disagreed with GAO's recommendations but said it would continue to evaluate prior authorization programs and take GAO's findings and recommendations into consideration in developing plans or determining appropriate next steps.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Centers for Medicare & Medicaid Services | The Administrator of CMS should subject accessories essential to the group 3 power wheelchairs in the permanent DMEPOS program to prior authorization. (Recommendation 1) |
CMS has taken steps to subject accessories essential to the group 3 power wheelchairs in the permanent DMEPOS program to prior authorization, as GAO recommended in April 2018. Specifically, CMS has begun a voluntary prior authorization program for certain group 3 power wheelchair accessories that are reviewed as part of prior authorization for the wheelchair bases. Allowing providers and suppliers to submit accessories essential to the group 3 power wheelchairs in the permanent program for prior authorization should enable CMS to achieve one of the stated benefits of prior authorization-to allow providers and suppliers to know prior to providing items whether Medicare will likely pay for them.
|
| Centers for Medicare & Medicaid Services |
Priority Rec.
The Administrator of CMS should take steps, based on results from evaluations, to continue prior authorization. These steps could include: (1) resuming the paused home health services demonstration; (2) extending current demonstrations; or, (3) identifying new opportunities for expanding prior authorization to additional items and services with high unnecessary utilization and high improper payment rates. (Recommendation 2) |
CMS has taken steps to evaluate and continue its prior authorization programs, as GAO recommended in April 2018. These steps have included (1) resuming the home health services demonstration with changes, (2) extending the end date of the repetitive scheduled non-emergency ambulance service demonstration, and (3) adding items to the required prior authorization list for the permanent program. CMS has also completed evaluations of prior authorization demonstrations and begun estimating cost savings from its actions. These steps should allow CMS to reduce unnecessary utilization and improper payments.
|
