Defense Health Agency: Oversight Needed to Better Ensure That Children Are Screened, Tested, and Treated for Lead Exposure
Fast Facts
The Defense Health Agency has developed pediatric lead screening, testing, and treatment guidelines for its military medical facilities. The guidance centers on screening and testing children for elevated blood lead levels and reporting results to appropriate authorities. But DHA doesn't oversee adherence to its guidelines.
DHA officials told us that they plan to conduct oversight, but didn't provide any documentation of its efforts or details about when its oversight would begin.
We recommended that DHA develop the oversight plan, including time frames, and implement it at its facilities.
Highlights
What GAO Found
The Defense Health Agency (DHA)—the agency responsible for managing military medical treatment facilities—developed standardized guidelines for facility providers on pediatric lead processes. These include screening and testing children for elevated blood lead levels, treating children with elevated levels as indicated, and reporting any confirmed elevated levels to the appropriate authorities. The guidelines state that facility providers should follow the Centers for Disease Control and Prevention (CDC) recommendations related to these pediatric lead processes. For example, the CDC recommends that children identified as having a high risk of exposure to lead be tested for elevated blood lead levels. DHA and military service officials told GAO they use email and other methods of communication to disseminate information about pediatric lead processes to facility providers, including DHA's new guidelines.
Pediatric Lead Processes Include:
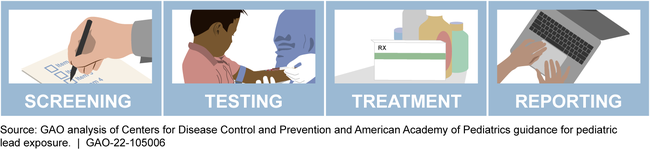
While DHA has developed pediatric lead guidelines and stated that it expects facility providers to follow them, DHA does not oversee facility providers' adherence to these guidelines. DHA officials told GAO that they intend to conduct oversight of pediatric lead screening, testing, treatment, and reporting of elevated blood lead levels by developing a dashboard using data elements from DHA's electronic health record system. However, DHA did not provide any documentation of these efforts or details such as a time frame for when this oversight will be implemented. The agency would be better positioned to ensure that the guidelines are consistently and systematically implemented across all facilities if DHA develops and implements a plan to oversee the pediatric lead processes.
In its 2021 report to Congress, DOD reported that 30,412 children were screened for lead exposure, 12,044 children were tested for elevated blood lead levels, and 83 children had elevated levels for the 8-month period covered. However, the data did not include pediatric lead screening and testing data from some facilities and complete information from others, likely representing an undercount. Further, DOD was unable to replicate the methodologies used to collect the data in the report. As a result, GAO could not determine the extent to which the data in the report were complete or if the data were accurate, and therefore reliable.
Why GAO Did This Study
The Department of Defense's (DOD) TRICARE program provides care to eligible pediatric beneficiaries through its military medical treatment facilities or civilian providers. The National Defense Authorization Act for Fiscal Year 2020 (NDAA 2020) required DOD to (1) establish guidelines for its facility providers on screening, testing, and reporting blood lead levels in children; (2) disseminate these guidelines to its facility providers; and (3) submit to Congress a report on the number of children screened for an elevated risk of lead exposure, tested for lead in the blood, and the number found to have an elevated blood lead level.
NDAA 2020 also included a provision for GAO to report on the effectiveness of DOD's pediatric lead processes. This report (1) describes the guidelines DHA established for facility providers for screening, testing, treating, and reporting of blood lead levels in children and how DOD disseminates them, (2) examines DHA oversight of facility provider adherence to the guidelines, and (3) describes the reliability—accuracy and completeness—of the data in DOD's report to Congress.
GAO reviewed relevant DOD guidelines; interviewed DHA and military service officials; and analyzed the reliability of DOD's report to Congress on pediatric lead.
Recommendations
GAO is recommending that DHA develop a plan, including time frames, to implement a process for overseeing military medical treatment facility providers' adherence to pediatric lead processes. DOD concurred with the recommendation.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Defense Health Agency | DHA should develop a plan, with time frames, to implement a process to oversee the extent to which MTF providers are adhering to DHA's guidelines related to pediatric lead screening, testing, treating, and reporting to ensure that these processes are implemented consistently across all MTFs. (Recommendation 1) |
When we confirm what actions the agency has taken in response to this recommendation, we will provide updated information.
|
