Laboratory Safety: FDA Should Strengthen Efforts to Provide Effective Oversight
Fast Facts
In 2017, the Food and Drug Administration created a new office within the agency to oversee its lab safety program. But, 3 years later, there are still disagreements within the FDA about the office's roles and responsibilities.
Moreover, FDA's lab safety program does not include key elements of effective oversight. For example, lab safety office staff cannot inspect labs unannounced. Unannounced inspections can help to better observe a lab's normal operating conditions.
We recommended that the FDA resolve disagreements over the office's roles and responsibilities and provide it with the authority to oversee lab safety.

Highlights
What GAO Found
The Food and Drug Administration (FDA) has taken steps intended to improve safety at its laboratories, including those that work with hazardous biological agents. Specifically, FDA created the Office of Laboratory Safety (OLS) in 2017 as a safety oversight body for all FDA laboratories.
Establishment of FDA's Office of Laboratory Safety (OLS)
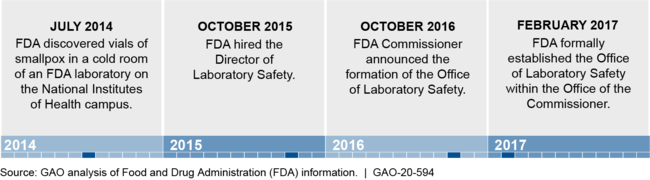
Note: Prior to March 2019, OLS was referred to as the Office of Laboratory Science and Safety.
In coordination with FDA's operating divisions—known as centers—OLS has standardized safety policies, incident reporting, inspections, and safety training. However in creating OLS, FDA did not implement key reform practices that could have helped ensure OLS's effectiveness. For example, FDA's centers and OLS did not reach a shared understanding of OLS's roles and responsibilities—a key practice for effective agency reforms. Although senior agency leaders were involved in developing OLS's strategic plan, disagreements about OLS's role raised by center directors at that time still remain. For example, center directors told GAO that OLS's mission should not include science, laboratory quality management, or inspections. Conversely, the director of OLS said OLS remains committed to its mission as envisioned in the strategic plan, which includes these areas of responsibility. FDA officials said they plan to update the plan in 2021, which presents an opportunity for FDA to address areas of disagreement.
In its current form, FDA's laboratory safety program also does not meet the key elements of effective oversight identified in GAO's prior work. For example,
- The oversight organization should have clear authority to ensure compliance with requirements. However, as part of a 2019 reorganization, FDA placed the OLS director at a lower level than the center directors. Also, OLS does not directly manage the center safety staff responsible for ensuring the implementation of safety policies that OLS develops. As a result, OLS has limited ability to access centers' laboratories—in part because they cannot inspect them unannounced—or to ensure compliance with safety policies.
- The oversight organization should also be independent from program offices to avoid conflict between program objectives and safety. However, OLS depends on the centers for much of its funding and has had to negotiate with the centers annually for those funds, which can allow center directors to influence OLS priorities through the funding amounts they approve. FDA has not assessed potential independence risks from using center funds for OLS. Without taking steps to do so, FDA's laboratory safety program will continue to compete with the centers' mission objectives and priorities.
Why GAO Did This Study
In 2014, FDA discovered improperly stored boxes of smallpox virus, posing a risk to individuals who might have been exposed. This raised concerns about the oversight of FDA's laboratories that conduct research on hazardous biological agents. In 2016, GAO made five recommendations to improve FDA's laboratory safety, four of which the Department of Health and Human Services (HHS) had not fully implemented as of July 2020.
GAO was asked to examine FDA's efforts to strengthen laboratory safety. This report examines FDA's efforts since GAO's 2016 report to improve safety in its laboratories that work with hazardous biological agents.
To conduct this work, GAO reviewed FDA documents; assessed FDA's safety oversight practices against key reform practices and oversight elements GAO identified in prior work; and interviewed FDA officials, including staff and senior leaders at OLS and the three centers that work with hazardous biological agents.
Recommendations
GAO is making five recommendations to FDA, including to resolve disagreements over roles and responsibilities, to provide OLS with the authority and access to facilities necessary to oversee laboratory safety, and to take steps to assess and mitigate any independence risks posed by how OLS is funded. HHS agreed with all five recommendations.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Food and Drug Administration |
Priority Rec.
The Commissioner of FDA should, as part of the agency's efforts to update OLS's strategic plan for overseeing agency-wide laboratory safety, resolve agency-wide disagreements on the roles and responsibilities for the centers and OLS in implementing laboratory safety reforms. (Recommendation 1) |
HHS agreed with and partially addressed our recommendation. In December 2020, FDA initiated a third-party review of its laboratory safety program, and the review was completed in March 2021. In keeping with our recommendation, the third-party reviewer recommended that FDA finalize the identification of roles and responsibilities for agency-wide safety initiatives. As of February 2022, FDA stated that its leadership and safety staff, through the existing Laboratory Science and Safety Council, were reviewing and developing updates to Staff Manual Guides related to FDA's safety program, including to further clarify laboratory safety roles and responsibilities across FDA's centers and offices. In fiscal year 2024, FDA combined several functions, including by merging the Office of Laboratory Safety with its occupational health office into the newly created Office of Occupational Health and Safety. Further, as of December 2025, FDA officials said that senior leadership was evaluating areas for consolidation and centralization across the agency to eliminate duplication, overlap, and fragmentation. Following this evaluation and any subsequent reorganization, FDA officials said they would develop a strategic plan and relevant Staff Manual Guides to guide the efforts of the Office of Occupational Health and Safety, to include critical elements for laboratory safety. We will continue to monitor FDA's efforts to resolve agency-wide disagreements on the roles and responsibilities for the centers and the Office of Occupational Health and Safety regarding oversight of laboratory safety.
|
| Food and Drug Administration | The Commissioner of FDA should, as part of the agency's efforts to update OLS's strategic plan for overseeing agency-wide laboratory safety, address issues of duplication, overlap, and fragmentation within the safety program. (Recommendation 2) |
HHS agreed with our recommendation, noting it launched a multi-phase evaluation of OLS. In December 2020, FDA initiated a third-party review of its laboratory safety program, and the review was completed in March 2021. In January 2023, FDA noted that the agency was updating documentation related to FDA's safety program to help address issues of duplication, overlap, and fragmentation. As of December 2025, FDA officials said senior leadership was evaluating areas for consolidation and centralization across the agency to eliminate duplication, overlap, and fragmentation. Following this evaluation and any subsequent reorganization, FDA officials said the agency will then develop a strategic plan and relevant Staff Manual Guides. We will continue to monitor FDA's efforts to address issues of duplication, overlap, and fragmentation within the laboratory safety program.
|
| Food and Drug Administration | The Commissioner of FDA should, as part of the agency's efforts to update OLS's strategic plan for overseeing agency-wide laboratory safety, identify how FDA leadership will communicate agency-wide on a sustained basis about the importance of laboratory safety and OLS's role in ensuring successful implementation of laboratory safety reforms. (Recommendation 3) |
HHS agreed with our recommendation, noting FDA intended to develop communication approaches that demonstrate a shared commitment to FDA laboratory safety. In March 2021, FDA officials reported that the review of the laboratory safety program discussed the development of a communication strategy to engage and inform stakeholder groups within the FDA laboratory safety program. FDA also shared communications that it disseminated to FDA staff to highlight the importance of laboratory safety, including most recently in March 2022. As of December 2025, FDA said that senior leadership was evaluating areas for consolidation and centralization across the agency. Following this evaluation and any subsequent reorganization, FDA officials said they would develop a strategic plan and relevant Staff Manual Guides. We will continue to monitor FDA's efforts to identify how FDA leadership will communicate agency-wide on a sustained basis about the importance of laboratory safety, including through a strategic plan or safety communication strategy for the laboratory safety program.
|
| Food and Drug Administration | The Commissioner of FDA should provide OLS—as FDA's laboratory oversight body—with the necessary oversight authority and access to laboratories to oversee FDA's laboratory safety program and ensure compliance with the agency's laboratory safety policies. (Recommendation 4) |
HHS agreed with this recommendation, and made policy changes that meet the intent of our recommendation. For example, in May 2024, FDA updated the staff manual guide for the Office of Occupational Safety and Health (formerly OLS) to note that responsibilities of its staff include conducting and overseeing laboratory practices and workplaces, including sufficient unannounced inspections. In March 2024, FDA revised its standard operating procedure outlining the OLS inspection process, and the Office of Occupational Safety and Health had performed 41 laboratory inspections across multiple centers since 2022, according to FDA in August 2025. As a result of these changes, FDA has provided the Office of Occupational Safety and Health with the necessary oversight authority and access to laboratories to ensure that the centers are fully complying with all laboratory safety policies.
|
| Food and Drug Administration | The Commissioner of FDA should take steps to assess and mitigate any risks to independence posed by funding OLS—as FDA's laboratory safety oversight body—through the working capital fund. In conducting its risk assessment, FDA should specifically focus on ensuring the decision-making processes of the working capital fund supports OLS's independence and ability to hire sufficient staff to implement its laboratory safety oversight priorities. (Recommendation 5) |
HHS agreed with our recommendation, noting it would continue to seek direct appropriations for OLS. HHS also stated that FDA's plan to update the OLS strategic plan would help the agency to develop a budget and staffing levels that were commensurate with OLS's duties. In May 2024, FDA reported that it received $5.6 million in appropriations for OLS for fiscal year 2023, an increase of $0.1 million over the amount appropriated in fiscal year 2023. OLS was also approved to receive $1.79 million from the working capital fund, which is funded by the centers and the Office of Regulatory Affairs that OLS oversees. In July 2025, FDA stated that the Office of Occupational Safety and Health (formerly OLS) continues to receive funding through direct congressional appropriations and the working capital fund. In December 2025, the agency stated it will continue to work to secure direct budget authority to support the full funding needed to for the comprehensive operations of the Office of Occupational Safety and Health, including to ensure its independence and ability to hire sufficient staff to effectively implement laboratory safety oversight efforts. We will continue to monitor FDA's efforts to implement this recommendation.
|
