Antibiotic Resistance: Additional Federal Actions Needed to Better Determine Magnitude and Reduce Impact
Fast Facts
Antibiotic-resistant infections can be difficult or impossible to treat.
We examined federal challenges to addressing antibiotic resistance:
Surveillance—Tracking resistant infections is tough because of reliance on sampling and voluntary reporting
Diagnostics—Lack of data on clinical outcomes impedes the development and use of antibiotic-resistance tests
New treatments—The pipeline of new antibiotics is inadequate and federal efforts haven’t fully incentivized development
Antibiotic use—Federal requirements to improve antibiotic use are mostly limited to hospitals and nursing homes
Our 8 recommendations are to strengthen federal efforts.
Pills spilling out of a prescription bottle
Highlights
What GAO Found
The precise magnitude of the problem of antibiotic resistance is unknown. The Centers for Disease Control and Prevention (CDC) has made progress in expanding surveillance of infections from certain antibiotic-resistant bacteria in the United States and abroad but faces several challenges.
2001-2017 Cumulative Spread of One Type of Highly Resistant Bacteria in the United States
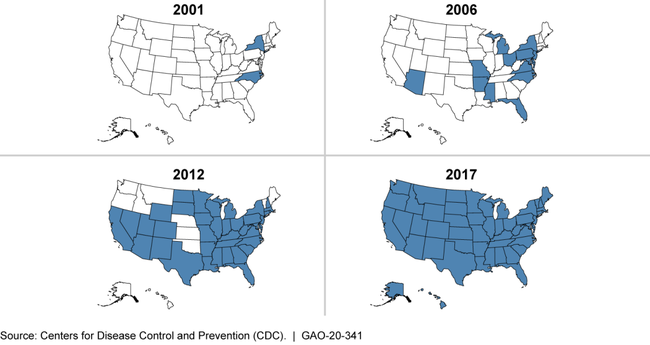
Note: This figure tracks a type of carbapenem-resistant Enterobacteriaceae (CRE), which, according to CDC, is a “nightmare bacteria” resistant to nearly all available antibiotics. Shading indicates CDC confirmed the presence of these bacteria within that state in that year or a previous one.
CDC faces challenges in conducting surveillance for antibiotic resistance due to the limited data it is able to collect through various surveillance systems. For example, CDC's primary surveillance system for gonorrhea—which CDC classified as an urgent antibiotic resistance threat affecting over half a million patients annually—currently tracks only an estimated 1 to 2 percent of all U.S. cases and only in males. CDC has not fully evaluated the representativeness of the gonorrhea surveillance system's results. However, it could do so, for example, by comparing the trends in their limited sample population with trends it can establish in the overall U.S. population via additional studies. Such an evaluation could give CDC more confidence that the system's data accurately reflect national trends.
Federal agencies have taken steps to advance the development and use of diagnostic tests to identify antibiotic-resistant bacterial infections, but these efforts have limitations. For example, agencies have conducted some studies to establish whether testing can lead to positive health care outcomes, such as reduced rates of antibiotic-resistant infections. However, more such studies are needed, according to experts and agency officials. Without information to guide test usage, clinicians may not be able to select appropriate treatments for their patients. One reason for the insufficient number of studies is that Department of Health and Human Services (HHS) agencies that are in a position to conduct or fund such studies—such as CDC and the Biomedical Advanced Research and Development Authority—disagree about what each agency should do. By clarifying roles and responsibilities, HHS agencies could more effectively address the need for more studies. The resulting studies could help demonstrate the value of diagnostic tests for antibiotic resistance, potentially increasing their use and improving patient care.
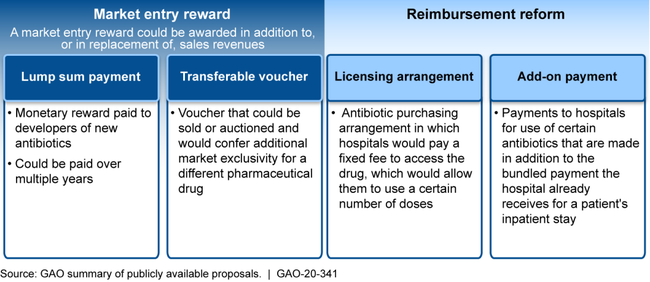
Experts warn that the current pipeline of antibiotics in development is insufficient to meet the threat of resistance. Several challenges impede the development of new treatments for resistant infections, notably inadequate return on investment for drug companies largely due to low prices and a limited patient population for whom these treatments would be appropriate. While HHS and Department of Defense agencies have provided financial premarket incentives to support antibiotic research and development, experts, federal officials and antibiotic developers agree that more postmarket incentives are needed to overcome the economic challenges. Advisory groups, including a presidential advisory council, and others have called for new postmarket incentives and identified multiple options for their design, including market entry rewards and reimbursement reform (see figure). However, HHS has not developed a strategy to further incentivize development of new treatments for antibiotic-resistant infections, and it may need to request authority and appropriations to create and implement certain types of incentives. Until such incentives are developed, more drug companies may exit the antibiotic development sector, and the pipeline of new treatments may continue to decrease.
Examples of Possible Postmarket Incentive Options to Encourage the Development of Antibiotics Identified by Advisory Groups and Others
Federal agencies have made several efforts to promote the appropriate use of antibiotics across health care settings through antibiotic stewardship—giving patients the right antibiotic at the right time, in the right dose, and for the right duration. However, key challenges remain. For example, federal agencies require only certain types of health care facilities to implement stewardship programs. In addition, CDC is limited in its ability to monitor and improve appropriate antibiotic use, in part because providers are not generally required to report antibiotic use data to a centralized database. The 5-year National Action Plan for Combating Antibiotic-Resistant Bacteria (National Action Plan) calls for strengthening antibiotic stewardship and for the timely reporting of antibiotic use data across health care settings. An executive order directs an interagency task force—the Combating Antibiotic-Resistant Bacteria (CARB) Task Force, coordinated by HHS—to provide annual updates to the President on, among other things, plans for addressing any barriers to full implementation of the National Action Plan. However, in its progress reports covering the first 4 years of the National Action Plan's implementation, the task force did not identify plans to address barriers to expanding antibiotic stewardship programs or the collection of antibiotic use data. Until it does so, the government will not have reasonable assurance that it is fully implementing the National Action Plan and addressing antibiotic resistance.
Why GAO Did This Study
Bacterial infections have become more difficult, and sometimes impossible, to treat due to antibiotic resistance, which occurs when bacteria develop the ability to defeat the available drugs designed to kill them. Concerns about rising rates of resistance to available treatment options prompted the federal government to create the 5-year National Action Plan in 2015. The plan called for federal agencies to strengthen surveillance, advance the development of diagnostic tests and new antibiotics, and slow the emergence of resistant bacteria, among other things.
GAO was asked to review federal efforts to address antibiotic resistance. This report examines federal efforts and challenges related to (1) surveillance of antibiotic resistance, (2) the development and use of diagnostic testing to identify antibiotic resistance, (3) the development of treatments for resistant infections, and (4) appropriate antibiotic use. GAO reviewed literature and agency documents; interviewed agency officials and health care industry, drug industry, and other stakeholders; and held a meeting of international and U.S. experts to obtain their views.
Recommendations
GAO is making eight recommendations to strengthen the federal response to combating antibiotic resistance. HHS concurred with seven recommendations and did not concur with one. More details are provided on the next page.
In response to the findings presented in this Highlights, GAO recommends that:
CDC ensure that its evaluation of its surveillance system for antibiotic-resistant gonorrhea includes measures of the system's representativeness of the U.S. population;
HHS identify leadership and clarify roles and responsibilities to assess the clinical outcomes of diagnostic testing;
HHS develop a strategy to further incentivize the development of new treatments for antibiotic-resistant infections, including through the use of postmarket financial incentives;
HHS direct the CARB Task Force to include in its annual updates to the President plans for addressing any barriers preventing full implementation of the National Action Plan.
In addition, GAO is making four recommendations to address other CDC efforts in surveillance and reporting and to address FDA efforts in monitoring diagnostic tests.
HHS did not concur with the recommendation that it develop a strategy that includes the use of postmarket financial incentives to encourage the development of new treatments for antibiotic-resistant infections, citing its ongoing analysis to understand whether postmarket incentives should be included in such a strategy. GAO recognizes the complexity of this issue and maintains that this recommendation is warranted given that experts and others have called for additional postmarket incentives and the insufficiency of the current pipeline of new treatments for antibiotic-resistant infections.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Centers for Disease Control and Prevention | The Director of CDC should take steps to determine participation rates and distribution needed in the AR Option of the National Healthcare Safety Network for conducting regional and national assessments of antibiotic resistance of public health importance. (Recommendation 1) |
The Department of Health and Human Services concurred with this recommendation. As of June 2021, CDC stated that it has worked to encourage voluntary reporting to the Antibiotic Use and Resistance module. CDC also noted that it seeks to extend geographic coverage of AR data and will include caveats about data representativeness in its reports. In April 2022, CDC told us they are continuing to work with CMS to explore options to increase reporting and encourage reporting to the Antibiotic Use and Reporting module. Finally, CDC noted that assessing representativeness was delayed due to the pandemic but will revisit this effort. On August 10, 2022, the Centers for Medicare and Medicaid Services finalized a rule in collaboration CDC to require reporting to the Antibiotic Use and Resistance Module by certain eligible hospitals. This rule represents a significant step forward in collecting more comprehensive data to enable assessments of antibiotic resistance of public health importance. However, there remain exemptions to required reporting that may limit the representativeness of regional or national assessments. In April 2023, CDC stated it expects to have the participation rates to conduct regional and national assessments of antimicrobial resistance in hospitals, once the rule is fully implemented, but have not provided any documentation to support that conclusion. In April, 2024, CDC stated it is taking steps to determine participation rates and distribution needed to assess the burden of antibiotic resistance. These steps include analyzing data from the Antibiotic Use and Resistance Module, supplemented with electronic health records data. Information CDC provided in December 2024 described the data sources and analysis it conducted to determine participation rates by geographical region. Based on the analysis, CDC concluded it had determined the participation rate sufficiency as offering more precision than previous to the Antibiotic Use and Resistance Module rule. CDC also described limitations to the reporting rate and effects on errors. We consider these steps to meet the intent of our recommendation.
|
| Centers for Disease Control and Prevention | The Director of CDC should ensure that CDC's evaluation of its surveillance system for antibiotic-resistant gonorrhea includes measures of its representativeness, such as comparison of the trends in the sample population with those in the overall U.S. population, using specially designed studies if needed. (Recommendation 2) |
The Department of Health and Human Services and the Centers for Disease Control and Prevention concurred with this recommendation. As of June 2021, CDC has taken some actions to address antibiotic-resistant gonorrhea, including publishing a report on a new type of resistant gonorrhea in the United States and evaluating new tests for resistant gonorrhea. In April 2022, CDC officials stated they were developing methods to create national estimates to account for possible biases within CDC's surveillance system, including constructing mathematical models to assess representativeness of surveillance data for both national and other populations of interest. In April 2023, CDC stated it recently published a paper on assessing the national representativeness of estimates of antimicrobial resistant gonorrhea in U.S. men. In April 2024, CDC stated it was preparing another journal article expanding the modeling analysis to incorporate women into its estimates. In December 2024, CDC stated that the modeling analysis described in April was complete and the manuscript is in preparation. In November 2025, CDC provided a citation to the published modeling analysis that accounted for representativeness of different subpopulations using a specially-designed study. The results of the study provide important information, including key limitations to their current surveillance data, about how antibiotic-resistant gonorrhea affects different groups, such as men and women. We believe this work meets the intent of the recommendation.
|
| Centers for Disease Control and Prevention | The Director of CDC should provide information on uncertainties for antibiotic resistance estimates in its consolidated Threats Reports, including standard errors or confidence intervals, as appropriate. (Recommendation 3) |
The Department of Health and Human Services generally concurred with this recommendation. As of June 2021, in a recent CDC publication on a variety of antibiotic-resistant infections in U.S. hospitals, data presented include confidence intervals and the report included discussion of key limitations in interpreting the data. However, CDC did not yet incorporate this sort of analysis into its consolidated Threats Reports, and the publication did not include information on all CDC-identified antibiotic resistant threats. Further, the publication is limited in scope to hospital patient infections and thus represents a subset of antibiotic resistant infections in the United States. In April 2022, CDC officials stated they were continuing discussions on the next consolidated Threats Report. They noted that efforts were dramatically impacted and delayed by the pandemic. In April 2024, CDC stated that they had notified all CDC reporting divisions that future data submissions and methodologies need to include confidence intervals and standard errors as appropriate. CDC was working to finalize timelines and methodologies for the next Threat Report. According to information CDC provided in December 2024, all contributing CDC divisions were notified of the requirement to include estimates of uncertainty as appropriate and are working to finalize timelines and methodologies for data submission. In November 2025, CDC sent a slide deck from a June 2025 presentation by CDC's Antimicrobial Resistance Coordination and Strategy Unit instructing data submitters that their estimates should include confidence or credible intervals. However, this information has not yet been included in an issued report. We will update the status of this recommendation as we receive additional information.
|
| Centers for Disease Control and Prevention | The Director of CDC should develop a plan for timely, consolidated reports of antibiotic resistance in priority pathogens at regular intervals. (Recommendation 4) |
The Department of Health and Human services concurred with this recommendation. As of June 2021, CDC stated that it was committed to updating the Threats Report at regular intervals and had begun planning for the next iteration. CDC noted that it will continue exploring reasonable timeframes for such reports given potential disruption such as the COVID-19 pandemic. In April 2022, CDC officials stated that planning was ongoing for the next report to be released in the coming years and that they plan to develop a more established plan for updates to this report. They were also assessing best options given impacts on antibiotic surveillance activities affected by the pandemic. In April 2023, CDC noted they published an interim report describing the impacts of the pandemic on antimicrobial resistance in the United States. In April 2024, CDC stated they were developing the infrastructure and timeline for regular electronic publication of estimates of antimicrobial resistance threats. CDC tentatively plans to publish pathogen-specific estimates as those become available through 2025, with a consolidated publication by the end of calendar year 2025. In December 2024, CDC stated that it plans to shift to a regular, 2-year reporting schedule, noting that there may be uncertainties based on data analyses and platform development factors. In November 2025, CDC provided a handout and a link to their web-page showing a plan to regularly issue reports every 2 years starting in 2026. We consider this to meet the intent of the recommendation.
|
| Office of the Secretary for HHS | The Secretary of HHS should identify leadership and clarify roles and responsibilities among HHS agencies to assess the clinical outcomes of diagnostic testing for identifying antibiotic-resistant bacteria. (Recommendation 5) |
HHS agreed with GAO's March 2020 recommendation and had taken some actions to implement it. As of December, 2021, HHS reported that its actions to address this recommendation included (1) introducing an objective within the diagnostic test goal of the 2020-2025 National Action Plan to support research into appropriate use of diagnostic tests; (2) designating the agencies (Agency for Healthcare Research and Quality, CDC, NIH, and the Military Infectious Diseases Research Program) assigned to this objective; and (3) coordinating activities, providing guidance, and compiling progress reports. However, the Combating Antibiotic-Resistant Bacteria Task Force stated it did not feel designating a single leader for these activities was warranted, relying instead on the agencies to conduct work based on their respective missions. In May 2022, HHS officials stated that the CARB Task Force provides leadership for the National Action Plan and that several teleconferences were held to communicate ongoing and upcoming work in which the agencies responsible for the relevant objective participated. In April 2023, HHS provided examples of activities conducted by several agencies to advance development and use of diagnostic tests. However, more specific documented details of how the agencies' mission translate into roles and responsibilities to address the objective can help agencies better manage fragmentation, which can help improve patient care and guide appropriate antibiotic use. We requested an update from the agency on this recommendation but, as of April 2025, had not received any additional information. We will update this recommendation as we receive more information.
|
| Other | The Commissioner of FDA should direct the Center for Devices and Radiological Health to conduct additional monitoring and evaluation of the status of FDA-authorized tests that rely on breakpoints, on a regular basis, to determine whether test manufacturers are updating breakpoints, seeking additional resources as needed. (Recommendation 6) |
The Department of Health and Human Services and the Food and Drug Administration concurred with this recommendation. FDA's Center for Devices and Radiological Health provided information regarding their initial effort to address this recommendation as of June 2021. FDA prepared and distributed a letter to manufacturers of tests relying on breakpoints encouraging the manufacturers to check the FDA website for updates and for guidance regarding when to submit an application to petition for changes to such tests. FDA stated this step represents a building block for monitoring and evaluating these tests. In June, 2022, FDA described steps taken to reduce the burden on test manufacturers to update breakpoints without necessarily needing a new 510(k) submission. CDRH within FDA noted that their resources were reallocated to address needs of the ongoing pandemic. In April 2023, FDA stated that CDRH took steps to identify currently marketed tests with outdated breakpoints and is in the process of drafting guidance about updating breakpoints in relevant labeling. In May, 2024, FDA provided an updated document (that went into effect September 29, 2023) that included guidance for facilitating breakpoint updates. For example, the guidance described use of a "Predetermined Change Control Plan" approach to encourage manufacturers to update their device breakpoints in device labeling. In July, 2025 FDA, provided information that it had monitored and evaluated the effects of the 2023 guidance and other steps described above, including by establishing and monitoring an inbox for receiving updated device labeling. FDA received label changes from most major test manufacturers. Additionally, FDA reported receiving product submissions that rely on the "Predetermined Change Control Plan" approach so that the test manufacturers no longer need to submit 510(k) submissions for breakpoint updates. FDA also reported continuing their outreach between their Centers and reviewing standards from external organizations. We consider these actions to meet the intent of the recommendation.
|
| Office of the Secretary for HHS | The Secretary of HHS should develop a strategic framework to further incentivize the development of new treatments for antibiotic-resistant infections, including through the use of postmarket financial incentives, and, if appropriate, make recommendations to Congress for necessary authority. (Recommendation 7) |
The Department of Health and Human Services did not concur with GAO's recommendation. In commenting on our report, the department noted that it has convened a work group to develop a strategic framework that includes proposals to address a variety of challenges facing antibiotic product developers and agreed that additional incentives are needed. However, the department stated it was still analyzing whether postmarket financial incentives should be included in this framework. As of June 2021, the department stated that it is committed to enhancing efforts to promote sustainability of the commercial market for new antibiotic products through the 2020-2025 CARB National Action Plan. Several HHS components conduct continuous monitoring and analysis of aspects of the antibiotics market to inform potential policy avenues. In May 2022, HHS officials told us they conducted a thorough analysis of issues relating to the need to address the limited pipeline for antibacterial products. Based on this analysis, HHS developed a draft strategic framework under consideration by HHS leadership. HHS also relied on this analysis to develop a legislative proposal that could create a novel payment mechanism to stimulate future innovation. In April 2023, HHS told us they continue to monitor and analyze the antimicrobial product pipeline and market challenges and recognize the post-market financial challenges facing developers. Additionally, the HHS FY2024 budget included funding to establish a novel payment mechanism that would delink revenue from sales volume for certain antimicrobial drugs. We consider HHS's strategic framework and documented approach for such funding to meet the intention of our recommendation and are closing the recommendation as implemented.
|
| Office of the Secretary for HHS | The Secretary of HHS should direct the CARB Task Force to include in its annual updates to the President plans for addressing any barriers preventing full implementation of the National Action Plan and, as appropriate, make recommendations for new or modified actions. Specifically, the CARB Task Force should identify plans to address barriers, such as those related to expanding (1) a CDC program designed to strengthen the U.S. response to resistant gonorrhea; (2) antibiotic stewardship programs across health care settings; and (3) antibiotic use data collection across health care settings, to the extent feasible. (Recommendation 8) |
The Department of Health and Human Services concurred with this recommendation, and stated that beginning in 2020 and continuing annually thereafter, the CARB Task Force's progress reports will include discussion of any barriers preventing full implementation of the National Action Plan, including, as appropriate, barriers that GAO has identified. Due to the COVID-19 pandemic, the CARB Task Force was delayed in developing the final progress report for the original National Action Plan. As of June 2021, it anticipates publishing the final progress report in fall 2021. In May, 2022, HHS officials provided a copy of the Final Progress report for the 2015-2020 National Action Plan which included discussion of barriers and plans to address such barriers. HHS officials also stated that all subsequent progress reports for the continuing National Action Plan (2020-2025) will discuss challenges and barriers as well as actions taken to address them. In July 2023, HHS issued their progress report for FY2021, which includes discussion on barriers, challenges, and ways to address some of the barriers and challenges. We consider this to meet the intention of the recommendation and are closing the recommendation as implemented.
|
