Prescription Opioids: Medicare Should Expand Oversight Efforts to Reduce the Risk of Harm
Fast Facts
What can Medicare do to help address the nation's opioid crisis?
We've previously found problems such as "doctor shopping" and questionable prescribing practices in Medicare's prescription drug benefits. With 14.4 million people receiving at least one opioid prescription through Medicare in 2016, reducing opioid overprescribing could make a difference.
We looked at Medicare's efforts to reduce inappropriate opioid prescribing, and we revisit our analysis in this testimony. We suggested ways to help Medicare collect information on doctor investigations and at-risk beneficiaries that could help it determine whether its efforts are working.
Photo of pills spilling out of a prescription drug bottle
Highlights
What GAO Found
The Centers for Medicare & Medicaid Services (CMS), within the Department of Health and Human Services (HHS), provides guidance on the monitoring of Medicare beneficiaries who receive opioid prescriptions to plan sponsors—private organizations that implement the Medicare drug benefit, Part D—but lacks information on most beneficiaries at risk of harm from opioid use.
CMS provides guidance to plan sponsors on how they should monitor opioid overutilization among Medicare Part D beneficiaries, and requires them to implement drug utilization review systems that use criteria similar to CMS's. CMS's criteria focused on beneficiaries who do all the following: (1) receive prescriptions of high doses of opioids, (2) receive prescriptions from four or more providers, and (3) fill prescriptions at four or more pharmacies. According to CMS, this approach focused actions on beneficiaries the agency determined to have the highest risk of harm.
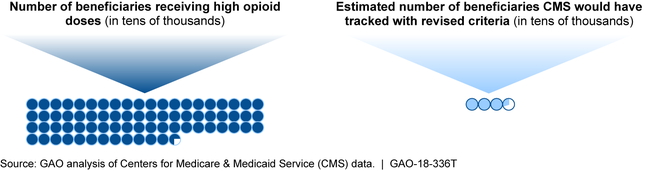
CMS's criteria, including recent revisions, do not provide sufficient information about the larger population of potentially at-risk beneficiaries. CMS estimates that while 33,223 beneficiaries would have met the revised criteria in 2015, 727,016 would have received high doses of opioids regardless of the number of providers or pharmacies. In 2016, CMS began to collect information on some of these beneficiaries using a higher dosage threshold for opioid use. This approach misses some who could be at risk of harm, based on Centers for Disease Control and Prevention guidelines. As a result, CMS is limited in its ability to assess progress toward meeting the broader goals of its Opioid Misuse Strategy for the Medicare and Medicaid programs, which includes activities to reduce the risk of harm to beneficiaries from opioid use.
CMS Estimates of 2015 Part D Beneficiaries with High Opioid Doses and Those Who Would Have Met Revised Overutilization Monitoring Criteria
CMS oversees the prescribing of drugs at high risk of abuse through a variety of projects, but does not analyze data specifically on opioids. According to CMS officials, CMS and plan sponsors identify providers who prescribe large amounts of drugs with a high risk of abuse, and those suspected of fraud or abuse may be referred to law enforcement. However, GAO found that CMS does not identify providers who may be inappropriately prescribing large amounts of opioids separately from other drugs, and does not require plan sponsors to report actions they take when they identify such providers. As a result, CMS is lacking information that it could use to assess how opioid prescribing patterns are changing over time, and whether its efforts to reduce harm are effective.
Why GAO Did This Study
Misuse of prescription opioids can lead to overdose and death. In 2016, over 14 million Medicare Part D beneficiaries received opioid prescriptions, and spending for opioids was almost $4.1 billion. GAO and others have reported on inappropriate activities and risks associated with these prescriptions.
This statement is based on GAO's October 2017 report (GAO-18-15) and discusses (1) CMS oversight of beneficiaries who receive opioid prescriptions under Part D, and (2) CMS oversight of providers who prescribe opioids to Medicare Part D beneficiaries. For the October 2017 report, GAO reviewed CMS opioid utilization and prescriber data, CMS guidance for plan sponsors, and CMS's strategy to prevent opioid misuse. GAO also interviewed CMS officials, the six largest Part D plan sponsors, and 12 national associations selected to represent insurance plans, pharmacy benefit managers, physicians, patients, and regulatory and law enforcement authorities.
Recommendations
In the October 2017 report, GAO made three recommendations that CMS (1) gather information on the full number of at-risk beneficiaries receiving high doses of opioids, (2) identify providers who prescribe high amounts of opioids, and (3) require plan sponsors to report to CMS on actions related to providers who inappropriately prescribe opioids. HHS concurred with the first two recommendations, but not with the third. GAO continues to believe the recommendation is valid, as discussed in the report and in this statement.
