COVID-19: FDA Took Steps to Help Make Tests Available; Policy for Future Public Health Emergencies Needed
Fast Facts
To help increase the availability of COVID-19 tests, the Food and Drug Administration didn't object when some labs used certain tests before they were reviewed for emergency use. Some of the unauthorized tests were eventually authorized, some were denied due to performance or data problems, and others were never reviewed by FDA. FDA began phasing out the use of unauthorized tests in November 2021.
But it's unclear how FDA would handle the use of unauthorized tests in a future public health emergency. We recommended developing a policy to help FDA make those decisions, particularly when enough authorized tests are available.
Highlights
What GAO Found
The Food and Drug Administration (FDA) took several actions aimed at increasing the availability of COVID-19 tests in the United States. This included granting emergency use authorizations (EUA) for more than 400 COVID-19 tests and sample collection devices by the end of 2021 (see figure). In a public health emergency, FDA may grant EUAs to temporarily allow the use of unapproved medical products, provided there is evidence that the product may be effective and that the known and potential benefits outweigh known and potential risks. FDA also exercised enforcement discretion for certain COVID-19 tests —that is, it did not object to laboratories' use of these COVID-19 tests before FDA had authorized them; this did not apply to tests that could be used at home. FDA's use of enforcement discretion helped increase test availability early in the pandemic.
Cumulative Number of COVID-19 Tests and Sample Collection Devices Authorized by FDA for Emergency Use, 2020-2021
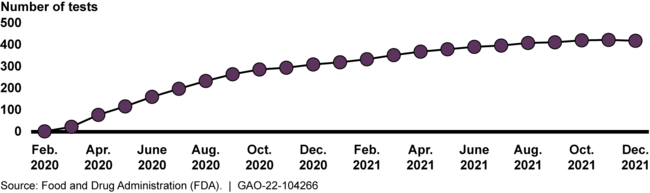
As of September 30, 2021, FDA had exercised its enforcement discretion for 370 tests. Test developers had submitted EUA requests for these tests, but FDA had not yet reviewed them. FDA officials told GAO they had concerns about the lack of review for these unauthorized tests, and as the number grew, the risks of this policy began to outweigh the benefits. Nevertheless, it was not until November 2021 that FDA updated its COVID-19 test policy with the intention of phasing out the agency's use of enforcement discretion and reducing the number of unauthorized tests. However, FDA has no policy for when it would begin and end exercising enforcement discretion for the use of unauthorized tests in a future public health emergency. Without such a policy, if FDA were to exercise similar enforcement discretion in the future, the agency could face the risk that tests with uncertain accuracy and reliability could be available for use for an extended period of time, even when a sufficient number of authorized tests are available. This could hamper an effective response and recovery during a crisis.
FDA monitors the performance of all COVID-19 tests—whether granted an EUA or not—through reports of performance problems submitted to FDA by test developers, health care providers, and consumers. According to FDA, this includes reports of false positive or false negative test results. By December 31, 2021, FDA had received more than 18,000 such reports for COVID-19 tests and took action to address identified problems. For example, FDA issued 10 letters to clinical laboratory staff and health care providers to inform them of safety concerns about COVID-19 tests.
Why GAO Did This Study
Diagnostic testing for COVID-19 is critical to tracking the virus, informing treatment, and suppressing transmission. However, because COVID-19 is caused by a novel virus, no test existed at the beginning of the pandemic. Typically, medical devices, such as diagnostic tests, must be approved or cleared by FDA before they can be offered. However, FDA's EUA authority requires a lower level of evidence than the effectiveness standard normally required for FDA product approval; therefore, it can help tests become available in a shorter amount of time. Test developers submit EUA requests to FDA that include data on a test's performance, and FDA reviews the data to determine whether to grant an EUA.
GAO was asked to review FDA's oversight of tests for COVID-19. This report examines, among other things, 1) the actions FDA took to help make COVID-19 tests available for use, 2) the number of tests FDA authorized and those for which it exercised enforcement discretion, and 3) FDA's monitoring of these tests after they were available for use. GAO reviewed agency documentation, and interviewed FDA and associations that represent test developers.
Recommendations
GAO recommends that FDA develop a policy for the use of enforcement discretion regarding unauthorized tests in future public health emergencies. This policy should include the conditions under which FDA would begin and end the use of such discretion. The Department of Health and Human Services concurred with our recommendation.
Recommendations for Executive Action
| Agency Affected Sort descending | Recommendation | Status |
|---|---|---|
| Food and Drug Administration | The Commissioner of FDA should develop a policy for the use of enforcement discretion regarding unauthorized tests in future public health emergencies. This policy should include the conditions under which FDA would begin and end the use of such discretion. (Recommendation 1) |
Open
FDA agreed with this recommendation and has begun taking action towards implementation. Specifically, HHS officials told us in April 2023 that FDA is in the process of developing a general policy for the use of enforcement discretion for unauthorized tests in public health emergencies, which will take into account lessons learned from both the COVID-19 and mpox public health emergencies. HHS noted that the policy will include the conditions under which FDA would begin and end the use of such discretion. Once FDA has developed this policy and shared it with us, we will review it to determine whether to close this recommendation.
|
