Drug Safety: FDA Should Take Additional Steps to Improve Its Foreign Inspection Program
Fast Facts
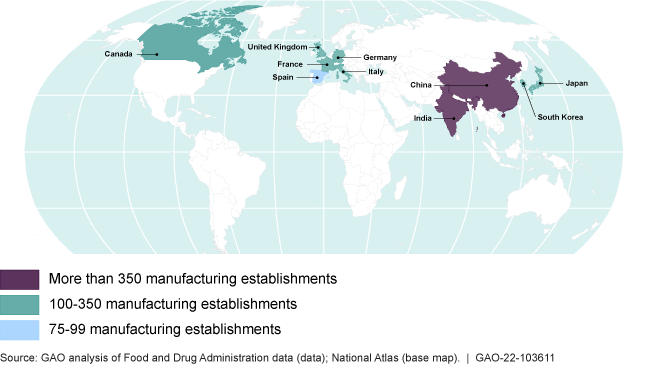
The Food and Drug Administration inspects foreign and domestic drug manufacturers to ensure drug safety and effectiveness. More than 50% of manufacturers supplying the U.S. market are located overseas—many in India and China.
The pandemic postponed almost all foreign inspections for most of 2020. But the number of these inspections had generally been declining since FY 2016—which FDA attributes in part to fewer investigators.
We recommended that FDA, among other things, develop tailored strategies and time frames to recruit and retain investigators for its foreign inspection workforce, which could help address a backlog of inspections.
The 10 countries with the most foreign drug establishments manufacturing drugs for the U.S. market as of June 2021
Highlights
What GAO Found
In fiscal year 2019, the Food and Drug Administration (FDA) began to increase the number of inspections of foreign drug manufacturing establishments after decreases from fiscal years 2016 through 2018. FDA, an agency within the Department of Health and Human Services (HHS), conducts the largest number of foreign inspections in India and China, where more than one-third of foreign establishments supplying the U.S. market are located. However, beginning in March 2020, FDA postponed most inspections because of the COVID-19 pandemic, conducting three foreign inspections from March to October 1, 2020. In comparison, FDA conducted more than 600 foreign inspections over the same time period in each of the 2 prior years. From October 2020 to April 2021 (the most recent period for which data are available), FDA conducted 18 high priority foreign inspections—primarily in China. In November 2021, FDA announced it was developing plans to potentially resume foreign inspections in February 2022.
GAO has reported that FDA faces unique challenges conducting foreign inspections—including that inspections have generally been preannounced and that investigators may rely on the establishment being inspected to provide translation services. While drugs manufactured overseas for the U.S. market must meet the same requirements as those manufactured in the U.S., these unique challenges raise questions about the equivalence of foreign to domestic inspections. FDA plans on implementing pilot programs focused on evaluating the effect of conducting unannounced inspections and using independent translation services. However, these efforts have been delayed by the COVID-19 pandemic and the agency has not yet finalized the pilots' designs.
As FDA moves forward, the agency could benefit from incorporating leading practices for designing a well-developed and documented pilot program—such as developing a methodology that details the information necessary to evaluate the pilot. This would help ensure the pilots provide FDA with the information it needs to assess the value of unannounced inspections and independent translation services, and to decide whether these approaches should be applied more broadly to other foreign inspections.
While FDA has reduced vacancies among its general drug inspection workforce, FDA data showed that the agency still has persistent vacancies among those who specialize in foreign inspections as of November 2021. Specifically:
- eight of 20 positions were vacant in FDA's cadre of drug investigators that conduct only foreign inspections, and
- five of 15 drug investigator positions were vacant in its foreign offices located in China and India.
These are longstanding challenges that GAO has previously identified. According to FDA officials, foreign inspection work is challenging, requiring the investigator to work independently in a foreign establishment under constrained time frames. In 2020 and 2021, FDA began to take steps to identify new strategies to recruit and retain this workforce, but the agency has not yet detailed implementation steps and time frames. Fully developing such tailored strategies could help ensure FDA has the workforce needed to meet its global mission.
Why GAO Did This Study
FDA is responsible for ensuring the safety and effectiveness of all drugs marketed in the U.S., regardless of where they are produced. Globalization—and the outbreak of COVID-19—have complicated FDA's oversight of the more than 4,000 establishments manufacturing drugs for the U.S. HHS reported that 73 percent of establishments manufacturing active ingredients, and 52 percent of those manufacturing finished drugs for the U.S., were located overseas as of March 2021.
GAO's concerns about FDA's ability to oversee the increasingly global drug supply chain led it to designate the issue as a high risk area in 2009. GAO was asked to update its work on FDA's foreign drug inspection program. This report (1) describes the number of inspections prior to and during the COVID-19 pandemic, (2) examines steps taken to address challenges related to preannouncing foreign inspections and language barriers, and (3) examines efforts to maintain a sufficient inspection workforce, among other objectives. For this work, GAO examined FDA data and documents and interviewed drug investigators and other FDA officials. GAO also visited FDA foreign offices in China and India in fall 2019.
Recommendations
GAO is making three recommendations: that FDA incorporate leading practices into the design of both its unannounced inspection and translation pilot programs and fully develop tailored strategies to ensure it has a sufficient foreign inspection workforce. HHS agreed with GAO's recommendations.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status Sort descending |
|---|---|---|
| Food and Drug Administration | The Commissioner of FDA should ensure the agency fully develops tailored strategies—including detailing implementation steps and time frames—focused on recruiting new and developing and retaining current investigators to specialize in conducting foreign drug inspections. (Recommendation 3) |
Open
HHS concurred with this recommendation, and in response to the recommendation FDA formed the GAO Recruitment and Retention Action Plan Work Group. In written responses from July 2022 through June 2023, FDA officials described six tailored strategies the work group had identified focused on recruitment, development, and retention of drug investigators who perform inspections overseas. The strategies include new efforts as well as further development of proposals we had described in our January 2022 report as being in the early stages. For example, FDA now offers investigators short-term details to the foreign drug cadre. As of June 2023, five drug investigators had been selected for 6-month details to the cadre and two drug investigators had been selected for 4-month details; three have since become permanent cadre members, according to FDA officials. As another example, FDA officials said that under an effort to recruit bilingual drug investigators, they hired one Mandarin/English speaker in July 2023. To fully implement this recommendation, FDA needs to provide documentation of the development of the six tailored strategies and their implementation in order to allow GAO to assess the extent to which they are fully tailored to recruit, develop, and retain foreign drug investigators. As part of our ongoing work examining FDA's foreign drug inspection program, we will continue to monitor and assess FDA's efforts. By implementing this recommendation FDA would be better able to ensure that it is recruiting investigators with an interest in foreign work and providing a pathway to cultivate its current investigators to gain the skills necessary to join the foreign cadre or foreign offices.
|
| Food and Drug Administration | The Commissioner of FDA should ensure that the agency incorporates leading practices we identified for designing a well-developed and documented pilot program as it finalizes its plans for implementing a pilot program to evaluate the effectiveness and efficiency of unannounced foreign inspections. (Recommendation 1) |
Closed – Implemented
HHS concurred with this recommendation. In October 2023, FDA provided the evaluation plan for the unannounced foreign inspection pilot program, which documented that FDA incorporated all five of our leading practices into the pilot's design. For example, the plan documented clear and measurable objectives and described a thorough assessment methodology. In a June 2023 response, FDA officials reported that the first phase of this pilot was implemented in India in March 2022, which was in line with the evaluation plan. FDA also provided an April 2023 evaluation of the first phase of the pilot in India, which was also in line with the evaluation plan and its leading practice of developing a plan to evaluate pilot results. FDA's actions meet the intent of our recommendation to incorporate leading practices into the pilot program to evaluate the effectiveness and efficiency of unannounced foreign inspections.
|
| Food and Drug Administration | The Commissioner of FDA should ensure that the agency incorporates leading practices we identified for designing a well-developed and documented pilot program as it finalizes its plans for implementing a pilot program to evaluate the costs and effects of using different types of translation services during foreign inspections. (Recommendation 2) |
Open – Partially Addressed
HHS concurred with this recommendation. In May 2023, FDA provided documentation detailing that, in response to GAO, it would incorporate all five of our leading practices into the design of the foreign inspection interpreter pilot program. For example, the documentation provided a list of measurable objectives and a data collection methodology. FDA officials told us that they plan to implement the pilot in China in 2024 and, in March 2024, provided an evaluation plan that referenced plans to incorporate all five of our leading practices into the pilot's design. However, FDA was not sure when in 2024 the interpreter pilot would begin, as it was prioritizing inspection resources for a different pilot--to examine unannounced inspections. This recommendation will remain open until FDA begins implementation to ensure it continues to incorporate the leading practices.
|
