Imported Seafood Safety: FDA Should Improve Monitoring of Its Warning Letter Process and Better Assess Its Effectiveness
Fast Facts
The Food and Drug Administration ensures that imported seafood is safe to eat. If companies violate food safety regulations and pose a public health risk, FDA may send them warning letters.
For the letters that FDA sent from 2014-early 2019, FDA didn't consistently follow key procedures or meet key goals. For example, FDA set a goal to conduct follow-up inspections within 6 months of issuing a warning letter to ensure violations were corrected. But FDA met this goal for just 14 of 125 warning letters sent for significant inspection violations.
We recommended that FDA monitor whether it's following procedures and meeting goals for its warning letters.
Highlights
What GAO Found
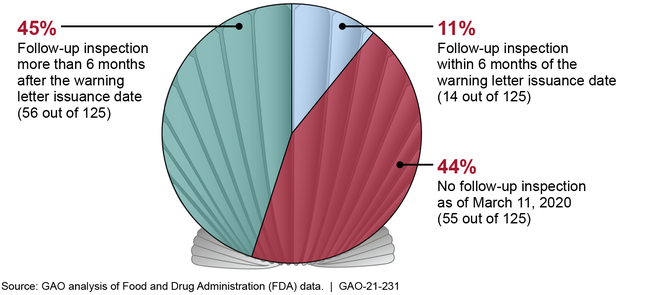
The Food and Drug Administration (FDA) issues warning letters for food safety violations that could pose a risk to public health. According to FDA, warning letters are its primary means of getting firms to voluntarily comply with food safety laws and regulations. GAO analyzed 167 imported seafood warning letters that FDA issued from January 1, 2014, through March 11, 2019, and found that FDA did not consistently follow key procedures or meet key goals for its warning letter process. For example, when FDA issues a warning letter based on significant inspection violations, the agency has a goal to conduct a follow-up inspection within 6 months of the date the warning letter was issued. Of the 167 warning letters we reviewed, 125 were based on significant inspection violations. FDA met its 6-month goal for 14 (11 percent) of these 125 letters. For 56 (45 percent) of these letters, FDA conducted a follow-up inspection more than 6 months after the warning letter was issued—on average, about 2 years. For the remaining 44 percent, FDA had not conducted a follow-up inspection, as of March 11, 2020.
Warning Letters Based on Significant Inspection Violations for Which FDA Met Its 6-Month Follow-up Inspection Goal, Issued January 1, 2014, Through March 11, 2019
While FDA has some monitoring tools, the agency does not have a monitoring process that allows it to determine whether all imported seafood warning letters (to both domestic and foreign firms) consistently follow procedures and meet goals, and FDA officials stated the agency had not conducted such a review of all letters. Developing a monitoring process, which could include regularly reviewing aggregate data, would increase FDA's awareness of whether the letters adhere to procedures and goals and help FDA ensure significant food safety violations have been adequately corrected.
FDA has not established performance goals and corresponding measures for its imported seafood warning letter process—key elements for assessing effectiveness. By developing performance goals and measures, such as percentage of warning letters resolved within 1 year of being issued, FDA would be better positioned to assess how well its process ensures the safety of imported seafood.
Why GAO Did This Study
FDA is responsible for ensuring the safety of most imported seafood. FDA relies, in part, on inspections of importers' facilities and of processors' foreign facilities to ensure compliance with federal law. If FDA identifies significant violations, such as firms not identifying food safety hazards likely to occur during processing, the agency can issue the firm a warning letter.
GAO was asked to review FDA's efforts to use warning letters to ensure the safety of imported seafood. This report examines the extent to which FDA (1) ensures it is following key procedures and meeting key goals for its warning letter process for imported seafood and (2) assesses the effectiveness of its warning letters in ensuring the safety of imported seafood. GAO reviewed FDA procedures and data and interviewed FDA officials.
Recommendations
GAO recommends that FDA (1) establish a process to monitor whether the agency is following the procedures and meeting the goals established for its warning letter process for imported seafood, and (2) develop performance goals and measures to assess how effective warning letters are at ensuring the safety of imported seafood. FDA agreed with GAO's recommendations.
Recommendations for Executive Action
| Agency Affected Sort descending | Recommendation | Status |
|---|---|---|
| Food and Drug Administration | The Commissioner of FDA should establish a process to monitor whether the agency is consistently following key procedures and meeting key goals for its imported seafood warning letters, and take corrective action when necessary. This could be done through regularly analyzing data that FDA collects, such as those in CMS and FACTS. (Recommendation 1) |
Open
FDA agreed with our recommendation that it establish a process to monitor whether the agency is consistently following key procedures and key goals for its imported seafood warning letters, and take corrective action when necessary. According to FDA, as of April 2023, the agency was developing a warning letter tracking dashboard that will be used to gather and analyze relevant data, such as those in CMS and FACTS, to enable the agency to focus on monitoring follow-up activity timeframes. In addition, FDA is working on updating a Field Management Directive to clarify the agency's goal for official action indicated (OAI) follow-up inspections and to ensure final OAI classifications are made in a timely manner. As of June 2023, FDA stated that it was continuing to work toward implementing a warning letter tracking dashboard and that it uses multiple tools to monitor warning letter follow-up activities in the meantime. Further, FDA updated Field Management Directive 086 to clarify the agency's goals for OAI follow-up inspections. Specifically, FDA clarified that the goal to complete a follow-up inspection within 6 months of an OAI classification applies to domestic inspections only. We will continue to follow up and report on any updates.
|
| Food and Drug Administration | The Commissioner of FDA should develop performance goals and measures to assess the effectiveness of its warning letters in ensuring the safety of imported seafood. Such measures could include, but need not be limited to, the percentage of warning letters cases that have been resolved, either through a closeout letter or import alert placement, within 1 year of being issued. (Recommendation 2) |
Open
FDA agreed with our recommendation to develop performance goals and measures to assess the effectiveness of its warning letters in ensuring the safety of imported seafood. As of April 2023, FDA stated that it was continuing to work on developing seafood-specific performance metrics, including seafood warning letters. Data related to imported food, foreign food suppliers, and importers are currently being published on the FDA Track Webpage, and the agency will provide an update when seafood-specific performance metrics have been developed. In a June 2023 update, FDA reiterated that it was continuing to work on developing seafood-specific performance metrics and was still publishing data on the FDA Track Webpage. FDA again stated that it will provide an update when seafood-specific performance metrics have been developed. We will continue to follow up and report on any updates.
|
