Federal Property: Formal Policies Could Enhance FDA's Property Management Efforts
Fast Facts
Three FDA regulatory centers help ensure the safety, security, and effectiveness of medical products. These centers had $200 million in property-related expenses in FY 2019.
We found that the centers did not consistently collect, analyze, and verify the accuracy of information needed to manage personal property, such as scientific and laboratory equipment and office furniture. Effective management of property can help ensure sound decision-making and minimize resource waste.
We made 4 recommendations, including that the FDA establish formal policies to use quality information to manage the personal property used by these centers.
Food and Drug Administration Laboratory Equipment

Highlights
What GAO Found
From fiscal years 2012 through 2019, the Food and Drug Administration (FDA) obligated a combined total of $14.7 billion to support operations at the Center for Drug Evaluation and Research, the Center for Biologics Evaluation and Research, and the Center for Devices and Radiological Health. During this period, 56 percent of the three centers' total obligations came from user fees, which FDA negotiates with and collects from regulated industries (e.g., manufacturers). The other 44 percent of the centers' total obligations came from regular appropriations. On average, property-related expenses represented 12 percent of the centers' total annual obligations; approximately half of property-related expenses were for rent.
Obligations for Three of the Food and Drug Administration's Regulatory Centers by Budget Authority Type, Fiscal Years 2012 through 2019
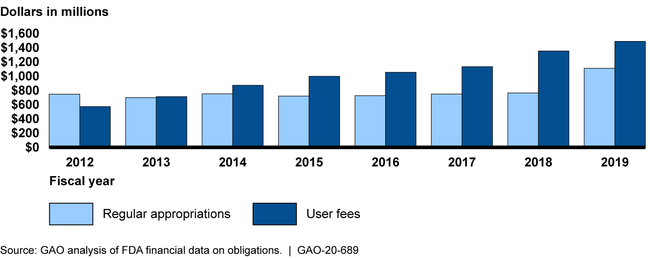
In managing their personal property (e.g., scientific equipment, computers, and office furniture), these centers did not consistently use quality information related to three phases of asset management:
(1) planning for property needs;
(2) operating and maintaining property; and
(3) reviewing property performance.
For example, officials at all three centers described informal, disparate processes for collecting and using information to identify and prioritize personal property needs. Furthermore, center staff conducted these activities differently, potentially resulting in inconsistent asset data. Using quality information—which involves consistently collecting, analyzing, and verifying the accuracy of data—can help agencies effectively manage assets such as personal property. It is a key characteristic integral to effective asset management, criteria GAO developed in prior work based on federal guidance and international standards. By establishing and implementing formal policies for using quality personal property information, FDA and the three centers can more effectively manage their personal property's useful life, plan for and respond to potential changes to the centers' funding and priorities, and maximize the value of the centers' personal property.
Why GAO Did This Study
Three FDA regulatory centers have primary responsibility for ensuring human medical products' safety, security, and effectiveness. FDA uses personal property and real property (e.g., buildings) to help achieve this oversight mission.
Congress included a provision in statute for GAO to examine FDA's expenses related to property at the three centers and evaluate FDA's ability to further its mission through management of those assets. Among other things, this report: (1) identifies the funds FDA obligated for these centers, and (2) assesses FDA's use of quality information to manage the centers' personal property. GAO reviewed financial data and interviewed officials about FDA's obligations from fiscal years 2012 through 2019. In addition, GAO compared criteria related to using quality information to FDA's relevant policies and processes, and interviewed FDA and General Services Administration (GSA) officials about FDA's property management.
Recommendations
GAO is making four recommendations to FDA and GSA, including that FDA establish and implement formal policies to use quality information to plan for, operate and maintain, and review performance of personal property used by the three centers. The agencies agreed with the recommendations.
Recommendations for Executive Action
| Agency Affected Sort descending | Recommendation | Status |
|---|---|---|
| Food and Drug Administration | The Commissioner of FDA should establish and implement formal policies to use quality information (e.g., linking decisions to mission-related goals) in the three centers' planning for their personal property needs, consistent with key characteristics integral to asset management leading practices. (Recommendation 1) |
Open
FDA concurred with this recommendation. In March 2024, FDA officials said that they intend to address this recommendation by having the Office of Laboratory Safety develop an agency-wide laboratory management strategy and roadmap. FDA officials said they plan to finalize the strategy and roadmap by December 2024. We will continue to monitor FDA's efforts to implement this recommendation.
|
| Food and Drug Administration | The Commissioner of FDA should establish and implement formal policies to use quality information (e.g., tracking condition, and maintenance and repair costs) in the three centers' operations and maintenance of personal property, consistent with key characteristics integral to asset management leading practices. (Recommendation 2) |
Open
FDA concurred with this recommendation. In March 2024, FDA officials said that they intend to address this recommendation by having the Office of Laboratory Safety develop an agency-wide laboratory management strategy and roadmap. FDA officials said they plan to finalize the strategy and roadmap by December 2024. We will continue to monitor FDA's efforts to implement this recommendation.
|
| Food and Drug Administration | The Commissioner of FDA should establish and implement formal policies to use quality information (e.g., measuring and documenting performance) in the three centers' reviews of personal property performance, consistent with key characteristics integral to asset management leading practices. (Recommendation 3) |
Open
FDA concurred with this recommendation. In March 2024, FDA officials said that they intend to address this recommendation by having the Office of Laboratory Safety develop an agency-wide laboratory management strategy and roadmap. FDA officials said they plan to finalize the strategy and roadmap by December 2024. We will continue to monitor FDA's efforts to implement this recommendation.
|
| General Services Administration | The Administrator of GSA should take steps to ensure that the condition of all White Oak facilities that FDA occupies are assessed, including limited access areas and tenant improvements that are above the standard services and facilities that GSA provides. (Recommendation 4) |
Closed – Implemented
The Food and Drug Administration (FDA) and General Services Administration (GSA) both play a role in keeping the office spaces and laboratories (i.e., real property) at FDA's White Oak campus in good working order so they can support the regulatory work performed there. In 2020, GAO reported that there were gaps in the condition assessments GSA uses to prioritize proposed repair and alteration projects at the White Oak campus. According to GSA officials, GSA's assessments did not include some areas such as certain laboratories due to restricted access to those spaces. Moreover, we found that GSA's building-engineering reviews did not assess the condition of some tenant improvements-such as epoxy flooring and paint. It is important that GSA, in its partner role with FDA, use quality information to manage real property, a key characteristic of effective property management that GAO identified in 2018. Therefore, GAO recommended the Administrator of GSA take steps to ensure that the condition of all White Oak facilities that FDA occupies are assessed, including limited access areas and tenant improvements that are above the standard services and facilities that GSA provides. In response to GAO's recommendation, in May 2021 GSA and FDA signed a memorandum of understanding to ensure GSA has access to all of FDA's White Oak campus facilities when it conducts its assessments. Specifically, the memorandum states that going forward the GSA Director onsite at FDA's White Oak campus will coordinate all inspections of the White Oak facilities with FDA's Director of the Office of Facility Management. With the new memorandum in place, GSA completed 13 building surveys at the White Oak campus between July 2021 and August 2021. By establishing clear roles and responsibilities to ensure unimpeded access during condition assessments and conducting the building surveys, GSA has closed the gaps GAO identified and met the intent of GAO's recommendation. With these changes, GSA and FDA may have greater confidence in the quality of information used to determine priorities and prepare for repair and replacement costs in offices and laboratories at the White Oak campus that are critical to FDA's mission.
|
