Foot-And-Mouth Disease: USDA's Evaluations of Foreign Animal Health Systems Could Benefit from Better Guidance and Greater Transparency
Fast Facts
Foot-and-mouth disease is highly contagious and affects certain livestock, including cattle. A domestic outbreak could cost the U.S. beef industry billions, and infected imported beef could lead to such an outbreak.
The U.S. Department of Agriculture evaluates the animal health systems of countries seeking to export beef to the U.S.—only approving those that they determine can assure disease-free beef.
Although USDA has a process for conducting such evaluations, it lacks detailed guidance for how its staff should document their analyses and results—leading to reliability and transparency concerns. We recommended USDA enhance its guidance.
Status of Foot-and-Mouth Disease Animal Health System Evaluations Conducted by USDA's Animal and Plant Health Inspection Service (APHIS), March 2017
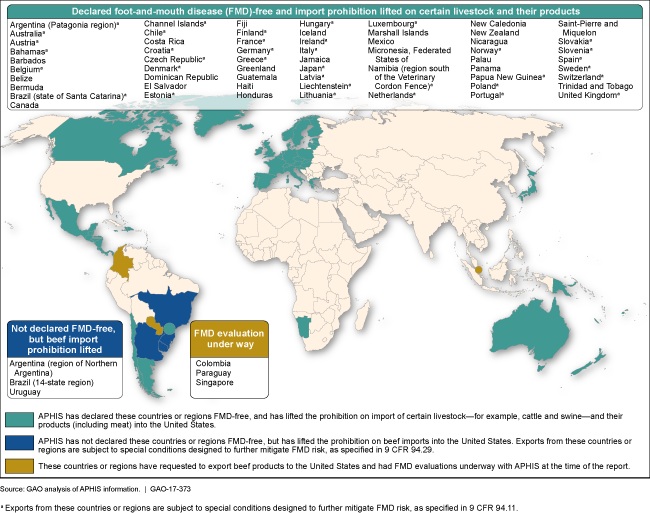
World map showing March 2017 status of USDA's animal health system evaluations.
Highlights
What GAO Found
The U.S. Department of Agriculture's (USDA) Animal and Plant Health Inspection Service's (APHIS) process for evaluating the animal health systems of countries seeking to export beef to the United States consists of five steps:
- A country requests that APHIS evaluate its animal health system.
- APHIS gathers information about the country's system, including documents identifying (1) veterinary control and oversight programs, (2) vaccination programs, (3) animal identification and movement controls, (4) laboratory diagnostic capabilities, and (5) animal-disease emergency-response measures.
- APHIS conducts in-country visits to verify and supplement this information.
- APHIS does a risk analysis to determine whether the country's beef products pose a risk to U.S. livestock and begins to draft a risk analysis report.
- APHIS determines an estimated risk level, which is included in the risk analysis report with a description of any mitigation measures the country must implement to ensure the safety of its beef exports. A report is completed and made public only for countries whose beef presents low risk. Countries whose beef poses a greater risk will not be eligible to export beef to the United States.
APHIS could strengthen its evaluation of foreign animal health systems by improving transparency to stakeholders, including the public. APHIS guidance instructs staff to adhere to timeframes for carrying out evaluations to ensure a lengthy process is completed efficiently. But the guidance does not instruct staff how to ensure evaluations are fully transparent. For example, APHIS guidance does not
- direct staff to document their analysis of country information and include all problems and concerns identified and how they were resolved;
- direct staff how to effectively document results of in-country visits, although the guidance requires these visits be documented; and
- indicate how to incorporate guidance on transparency from USDA's Chief Information Officer and the Office of Management and Budget into final risk analysis reports.
Without sufficient guidance instructing staff to document such items, it is unclear (1) how APHIS verifies country information and assesses its reliability; (2) how problems identified are ultimately addressed to APHIS's satisfaction; and (3) what methodologies, sources, assumptions, and uncertainties may influence its risk analysis. Further, according to the World Organisation for Animal Health, because risk analysis is inherently subjective, the process must be documented transparently. During GAO's review, APHIS acknowledged the weaknesses in its guidance and formed a team to begin work to address them. By completing this effort, APHIS may be better able to ensure that it has assessed risks fairly and consistently across countries and over time, and that the process is transparent to the public and other stakeholders.
Why GAO Did This Study
Foot-and-mouth disease (FMD) is a virus that causes painful lesions, making it difficult for livestock to stand or eat and greatly reducing meat and milk production. No FMD cases have been recorded in the United States since 1929. Federal regulations restrict fresh beef imports from countries where the disease is present because the disease may survive in untreated, uncooked beef (beef), and can be costly to control and eliminate. According to USDA, an outbreak of FMD could cause grave damage to the U.S. beef industry, which had a retail value of $95 billion in 2014.
GAO reviewed (1) USDA's process for evaluating the animal health systems of countries seeking to export beef products to the United States, and (2) how this process could be improved. GAO analyzed documentation supporting seven countries' requests for FMD animal health system evaluations. GAO also reviewed federal regulations, guidance, and a key trade agreement; and interviewed knowledgeable USDA and industry officials.
Recommendations
GAO is making three recommendations including that USDA clarify its guidance on how staff should document analysis of a foreign country's animal health system and the results of in-country visits to verify information. USDA agreed with GAO's recommendations and described actions it is taking or plans to take to implement them.
Recommendations for Executive Action
| Agency Affected Sort descending | Recommendation | Status |
|---|---|---|
| Department of Agriculture | To improve USDA's evaluations of foreign countries' animal health systems, the Secretary of Agriculture should direct the Administrator of APHIS to develop guidance promoting greater transparency in risk analysis reports in accordance with the quality information guidelines issued by USDA's Chief Information Officer and guidance from the Office of Management and Budget. |
Closed – Implemented
In August 2017, APHIS updated its "Guidelines for Conducting Animal Health Status Evaluations." The guidelines state that APHIS staff are to "adhere to the general quality standards and information quality criteria developed by the Office of the Chief Information Officer (OCIO) for USDA agencies, and units that develop and review information and disseminate it to the public." The updated guidelines also provide a link to the OCIO standards. Further, the guidelines state that staff should conduct evaluations in accordance with guidance provided by the Office of Management and Budget in a memorandum titled "Updated Principles for Risk analysis," in which OMB describes the expectations and principles for government agencies with regard to risk assessments and risk communication. These actions implement GAO's recommendation.
|
| Department of Agriculture | To improve USDA's evaluations of foreign countries' animal health systems, the Secretary of Agriculture should direct the Administrator of APHIS to complete its efforts to develop agency guidance, clarifying that (1) staff must document, separately from the final risk analysis report, how key information gathered about a foreign country's animal health system was analyzed and how the information supports each of eight evaluation factors, and (2) in-country site visits must be appropriately and consistently documented in trip reports and should detail verification activities |
Closed – Implemented
In August 2017, APHIS reported that it had issued in March 2017 "Guidance for the Application of the 8 Factors for Animal Health Status Evaluations of Foreign Regions." The guidance includes a document tool to allow the lead staff officer to record findings from the evaluation while engaged in the assessment. This tool allows staff to document how they determine an applicant has met the standard for each of the 8 factors. In addition, in March 2017 APHIS's director of Regional Evaluation Services issued a directive requiring staff to use the new guidance and analytical tool in the future. APHIS also has reported that it will assess by the end of calendar year 2017 whether and how well staff are using the new guidance. APHIS also in August 2017, issued updated "Guidelines for Conducting Animal health Status (Regional) Evaluations" indicating that site visit reports are to be prepared immediately after trip completion and completed within 28 days. These steps implement GAO's recommendation.
|
| Department of Agriculture | To improve USDA's evaluations of foreign countries' animal health systems, the Secretary of Agriculture should direct the Administrator of APHIS to complete its efforts to develop an information management system to better store, organize, and manage documentation gathered about a foreign country's animal health system. |
Closed – Implemented
APHIS implemented a Projecting Tracking System to store and document information for different region evaluations in January 2017. According to APHIS, all active evaluations were moved into the system by April 30 2017. The software platform for this information is the new RES SharePoint site. APHIS provided GAO with a screenshot of the new SharePoint information management site, which appears to provide a storage, organizing, and document management system for information collected about foreign animal health systems. The implementation of this project tracking system implements GAO's recommendation.
|
