Drug Shortages: Public Health Threat Continues, Despite Efforts to Help Ensure Product Availability
Highlights
What GAO Found
The number of drug shortages remains high. Although reports of new drug shortages declined in 2012, the total number of shortages active during a given year—including both new shortages reported and ongoing shortages that began in a prior year—has increased since 2007. Many shortages are of generic sterile injectable drugs. Provider association representatives reported that drug shortages may force providers to ration care or rely on less effective drugs.
Number of Active Drug Shortages from January 2007 through June 2013
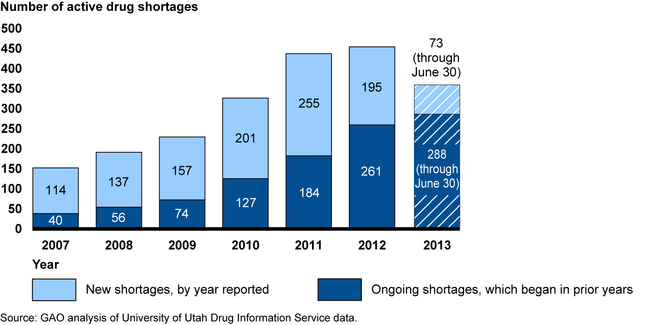
The immediate cause of drug shortages can generally be traced to a manufacturer halting or slowing production to address quality problems, triggering a supply disruption. Other manufacturers have a limited ability to respond to supply disruptions due to constrained manufacturing capacity. GAO's analysis of data from the Food and Drug Administration (FDA) also showed that quality problems were a frequent cause. GAO also identified potential underlying causes specific to the economics of the generic sterile injectable drug market, such as that low profit margins have limited infrastructure investments or led some manufacturers to exit the market.
While shortages have persisted, FDA has prevented more potential shortages in the last 2 years by improving its responsiveness. Among other things, FDA implemented Food and Drug Administration Safety and Innovation Act (FDASIA) requirements and recommendations GAO made in 2011. FDA has also initiated other steps to improve its response to shortages, such as developing procedures to enhance coordination between headquarters and field staff. However, there are shortcomings in its management of drug shortage data that are inconsistent with internal control standards. For example, FDA has not created policies or procedures governing the management of the data and does not perform routine quality checks on its data. Such shortcomings could ultimately hinder FDA's efforts to understand the causes of specific shortages as well as undermine its efforts to prevent them from occurring. In addition, FDA has not conducted routine analyses of the data to proactively identify and evaluate the risks of drug shortages.
Why GAO Did This Study
From prolonged duration of a disease, to permanent injury, to death, drug shortages have led to harmful patient outcomes. FDA—an agency within the Department of Health and Human Services (HHS)—is responsible for protecting public health and works to prevent, alleviate, and resolve shortages. In 2011, GAO recommended that FDA should enhance its ability to respond to shortages. In 2012, FDASIA gave FDA new authorities to improve its responsiveness and mandated GAO to study drug shortages.
In this report, GAO (1) reviews the trends in recent drug shortages and describes what is known about their effect on patients and providers; (2) examines the causes of drug shortages; and (3) evaluates the progress FDA has made in addressing drug shortages. GAO analyzed data from FDA and the University of Utah Drug Information Service, which is generally regarded as the most comprehensive source of drug shortage information for the time period we reviewed. GAO interviewed officials from FDA and other federal agencies, organizations representing patients and providers, and drug manufacturers. GAO also reviewed the literature, relevant statutes, regulations, and documents.
Recommendations
FDA should strengthen its internal controls over its drug shortage data and conduct periodic analyses to routinely and systematically assess drug shortage information, using this information to proactively identify drug shortage risk factors. HHS agreed with GAO's recommendations.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status Sort descending |
|---|---|---|
| Food and Drug Administration | To enhance its oversight of drug shortages, particularly as the agency fine-tunes the manner in which it gathers data on shortages and transitions from its database to a more robust system, the Commissioner of FDA should develop policies and procedures for the use of the existing drug shortages database (and, ultimately, the new drug shortages information system) to ensure staff enter information into the database in a consistent manner and to ensure the accuracy of the information in the database. |
Closed – Implemented
In July 2016, FDA shared documentation outlining procedures for entering information in to its recently established "Shortage Tracker." Specifically, FDA provided three step-by step guides covering a variety of topics including creating and updating drug shortage notifications and concerns. There is also a feature to help FDA prepare its annual drug shortage report to Congress. In addition, FDA provided training materials developed by the contractor that created the Shortage Tracker. The training, which was given in March 2016--the month FDA began using the Shortage Tracker--included topics such as entering data consistently, uploading supporting documentation and searching for historical information, and how to determine the statuses of abbreviated new drug application that are being considered for expedited review to help address a shortage.
|
| Food and Drug Administration | To enhance its oversight of drug shortages, particularly as the agency fine-tunes the manner in which it gathers data on shortages and transitions from its database to a more robust system, the Commissioner of FDA should conduct periodic analyses using the existing drug shortages database (and, eventually, the new drug shortages information system) to routinely and systematically assess drug shortage information, and use this information proactively to identify risk factors for potential drug shortages early, thereby potentially helping FDA to recognize trends, clarify causes, and resolve problems before drugs go into short supply. |
Open – Partially Addressed
In September 2018, FDA told us that it was using its drug shortage data system, the "Shortage Tracker," to summarize information reported by manufacturers as the reasons for existing shortages. The agency indicated that it was developing a model that would help identify early indicators that may predict future shortages. In June 2021, the agency reported that it encountered several challenges with directly predicting drug shortages while working on its initial modeling, including a lack of information on several critical elements of the drug supply chain. In a June 2023 written response, FDA indicated that it determined that a modeling effort to directly predict drug shortages would not be feasible with the available data. However, in October 2023, FDA officials said the agency had developed data systems that would provide staff, including drug shortages staff, with more accessible information about manufacturing supply chains and could be used to identify signals that could lead to a supply disruption, and a subsequent drug shortage. They said these systems would be fully implemented over the next year. GAO will leave this recommendation open until FDA fully implements this system for routine use.
|
