Laboratory Safety: FDA Should Strengthen Efforts to Provide Effective Oversight
Fast Facts
In 2017, the Food and Drug Administration created a new office within the agency to oversee its lab safety program. But, 3 years later, there are still disagreements within the FDA about the office's roles and responsibilities.
Moreover, FDA's lab safety program does not include key elements of effective oversight. For example, lab safety office staff cannot inspect labs unannounced. Unannounced inspections can help to better observe a lab's normal operating conditions.
We recommended that the FDA resolve disagreements over the office's roles and responsibilities and provide it with the authority to oversee lab safety.

Highlights
What GAO Found
The Food and Drug Administration (FDA) has taken steps intended to improve safety at its laboratories, including those that work with hazardous biological agents. Specifically, FDA created the Office of Laboratory Safety (OLS) in 2017 as a safety oversight body for all FDA laboratories.
Establishment of FDA's Office of Laboratory Safety (OLS)
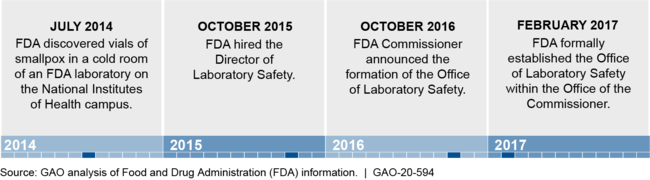
Note: Prior to March 2019, OLS was referred to as the Office of Laboratory Science and Safety.
In coordination with FDA's operating divisions—known as centers—OLS has standardized safety policies, incident reporting, inspections, and safety training. However in creating OLS, FDA did not implement key reform practices that could have helped ensure OLS's effectiveness. For example, FDA's centers and OLS did not reach a shared understanding of OLS's roles and responsibilities—a key practice for effective agency reforms. Although senior agency leaders were involved in developing OLS's strategic plan, disagreements about OLS's role raised by center directors at that time still remain. For example, center directors told GAO that OLS's mission should not include science, laboratory quality management, or inspections. Conversely, the director of OLS said OLS remains committed to its mission as envisioned in the strategic plan, which includes these areas of responsibility. FDA officials said they plan to update the plan in 2021, which presents an opportunity for FDA to address areas of disagreement.
In its current form, FDA's laboratory safety program also does not meet the key elements of effective oversight identified in GAO's prior work. For example,
- The oversight organization should have clear authority to ensure compliance with requirements. However, as part of a 2019 reorganization, FDA placed the OLS director at a lower level than the center directors. Also, OLS does not directly manage the center safety staff responsible for ensuring the implementation of safety policies that OLS develops. As a result, OLS has limited ability to access centers' laboratories—in part because they cannot inspect them unannounced—or to ensure compliance with safety policies.
- The oversight organization should also be independent from program offices to avoid conflict between program objectives and safety. However, OLS depends on the centers for much of its funding and has had to negotiate with the centers annually for those funds, which can allow center directors to influence OLS priorities through the funding amounts they approve. FDA has not assessed potential independence risks from using center funds for OLS. Without taking steps to do so, FDA's laboratory safety program will continue to compete with the centers' mission objectives and priorities.
Why GAO Did This Study
In 2014, FDA discovered improperly stored boxes of smallpox virus, posing a risk to individuals who might have been exposed. This raised concerns about the oversight of FDA's laboratories that conduct research on hazardous biological agents. In 2016, GAO made five recommendations to improve FDA's laboratory safety, four of which the Department of Health and Human Services (HHS) had not fully implemented as of July 2020.
GAO was asked to examine FDA's efforts to strengthen laboratory safety. This report examines FDA's efforts since GAO's 2016 report to improve safety in its laboratories that work with hazardous biological agents.
To conduct this work, GAO reviewed FDA documents; assessed FDA's safety oversight practices against key reform practices and oversight elements GAO identified in prior work; and interviewed FDA officials, including staff and senior leaders at OLS and the three centers that work with hazardous biological agents.
Recommendations
GAO is making five recommendations to FDA, including to resolve disagreements over roles and responsibilities, to provide OLS with the authority and access to facilities necessary to oversee laboratory safety, and to take steps to assess and mitigate any independence risks posed by how OLS is funded. HHS agreed with all five recommendations.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Food and Drug Administration |
Priority Rec.
The Commissioner of FDA should, as part of the agency's efforts to update OLS's strategic plan for overseeing agency-wide laboratory safety, resolve agency-wide disagreements on the roles and responsibilities for the centers and OLS in implementing laboratory safety reforms. (Recommendation 1) |
Open – Partially Addressed
HHS agreed with and partially addressed our recommendation. In December 2020, FDA initiated a third-party review of its laboratory safety program, and the review was completed in March 2021. In keeping with our recommendation, the third-party reviewer recommended that FDA finalize the identification of roles and responsibilities for agency-wide safety initiatives. As of February 2022, FDA stated that its leadership and safety staff, through the existing Laboratory Science and Safety Council, were reviewing and developing updates to Staff Manual Guides related to FDA's safety program, including to further clarify laboratory safety roles and responsibilities across FDA's centers and offices. As of January 2023, FDA stated that it is developing documents outlining the roles and responsibilities of the components within FDA's safety program. According to FDA, these documents will be shared with the FDA Commissioner and other FDA leadership for review and approval. In February 2024, FDA stated that it plans to complete these updates by the end of the year. These documents will inform the agency in its update to the OLS strategic plan. We will update the status of this recommendation when we receive additional information regarding the finalization and implementation of these documents, including an updated OLS strategic plan.
|
| Food and Drug Administration | The Commissioner of FDA should, as part of the agency's efforts to update OLS's strategic plan for overseeing agency-wide laboratory safety, address issues of duplication, overlap, and fragmentation within the safety program. (Recommendation 2) |
Open – Partially Addressed
HHS agreed with our recommendation, noting it launched a multi-phase evaluation of OLS. In December 2020, FDA initiated a third-party review of its laboratory safety program, and the review was completed in March 2021. As of January 2023, FDA noted that the agency is updating documentation related to FDA's safety program to help address issues of duplication, overlap, and fragmentation. In February 2024, FDA stated that it plans to complete these updates by the end of the year. These documents will inform the basis development of an updated OLS strategic plan. We will update the status of this recommendation when we receive additional information on FDA's efforts, including an updated OLS strategic plan.
|
| Food and Drug Administration | The Commissioner of FDA should, as part of the agency's efforts to update OLS's strategic plan for overseeing agency-wide laboratory safety, identify how FDA leadership will communicate agency-wide on a sustained basis about the importance of laboratory safety and OLS's role in ensuring successful implementation of laboratory safety reforms. (Recommendation 3) |
Open – Partially Addressed
HHS agreed with our recommendation, noting FDA intended to develop communication approaches that demonstrate a shared commitment to FDA laboratory safety. In March 2021, FDA officials reported that the review of the laboratory safety program discussed the development of a communication strategy to engage and inform stakeholder groups within the FDA laboratory safety program. FDA also shared communications that it disseminated to FDA staff to highlight the importance of laboratory safety, including most recently in March 2022. As of June 2023, FDA stated they continue to take interim steps that will help inform the development of a strategy for agency-wide communications on the importance of laboratory safety, such as sharing a message from the Chief Scientist with FDA staff on the agency's commitment to laboratory safety . We will update the status of this recommendation when we receive additional information regarding FDA's efforts to implement this recommendation, including through an updated OLS strategic plan or through the development of a safety communication strategy.
|
| Food and Drug Administration | The Commissioner of FDA should provide OLS—as FDA's laboratory oversight body—with the necessary oversight authority and access to laboratories to oversee FDA's laboratory safety program and ensure compliance with the agency's laboratory safety policies. (Recommendation 4) |
Open
HHS agreed with this recommendation, but stated that OLS already has the necessary authority to oversee the laboratory safety program and ensure compliance. In August 2021, FDA reported that OLS plans to inspect all high-containment and select agent laboratories annually, and all center laboratories triennially beginning in calendar year 2022. In August 2022, FDA reported that OLS started this inspection process in March 2022. As of June 2023, FDA stated the agency will further clarify OLS responsibilities, including conducting unannounced inspections, an important oversight inspection tool. FDA did not address how it ensures OLS has access to center laboratories. Until FDA provides these details on OLS's authority to oversee FDA's laboratory safety programs and the necessary access to laboratories to ensure compliance, FDA will lack assurance that all of FDA's laboratories are fully complying with laboratory safety policies. We will update the status of this recommendation when we receive additional information regarding FDA's efforts to implement this recommendation.
|
| Food and Drug Administration | The Commissioner of FDA should take steps to assess and mitigate any risks to independence posed by funding OLS—as FDA's laboratory safety oversight body—through the working capital fund. In conducting its risk assessment, FDA should specifically focus on ensuring the decision-making processes of the working capital fund supports OLS's independence and ability to hire sufficient staff to implement its laboratory safety oversight priorities. (Recommendation 5) |
Open
HHS agreed with our recommendation, noting it would continue to seek direct appropriations for OLS. HHS also stated that FDA's plan to update the OLS strategic plan would help the agency to develop a budget and staffing levels that were commensurate with OLS's duties. As of January 2023, FDA reported that it requested $9.1 million in appropriations for OLS for fiscal year 2023. In June 2023, FDA reported that it received $5.5 million in appropriations for OLS for fiscal year 2023, an increase of $1.5 million over the amount appropriated in fiscal year 2022. OLS was also approved to receive $1.75 million from the working capital fund, which is funded by the centers and the Office of Regulatory Affairs that OLS oversees. FDA stated it continues to work with the Administration to determine the best sources of funding to ensure OLS can accomplish its mission with independence. However, we remain concerned that if this request does not result in the necessary funding for OLS, FDA will continue to rely on funding from the centers and the Office of Regulatory Affairs, which has the potential to influence OLS safety priorities and thus impede OLS's independence, as we noted in our report. We maintain that FDA needs to assess and mitigate potential risks to independence posed by funding OLS through the working capital fund. We will update the status of this recommendation when we receive additional information regarding FDA's efforts to implement this recommendation.
|
