Food Safety: Additional Actions Needed to Help FDA's Foreign Offices Ensure Safety of Imported Food
Highlights
What GAO Found
The Food and Drug Administration's (FDA) foreign offices have engaged in a variety of activities since 2010 to help ensure that imported food is safe. Foreign offices reported that building relationships with foreign counterparts and gathering and assessing information were among their top priorities. As directed by the FDA Food Safety Modernization Act (FSMA), foreign offices also inspected foreign food facilities. Under FSMA, FDA is to inspect at least 600 foreign food facilities in 2011 and, for each of the next 5 years, inspect at least twice the number of facilities inspected during the previous year. As shown in the figure below, FDA is not currently keeping pace with the FSMA mandate. FDA officials told GAO that they do not plan to meet the FSMA mandate because of funding, and they question the usefulness of conducting that many inspections. However, FDA has not conducted an analysis to determine whether the number of inspections in the FSMA mandate or the lower number of inspections it is conducting is sufficient to ensure comparable safety of imported and domestic food. Without such an analysis, FDA is not in a position to know what is a sufficient number of foreign inspections and, if appropriate, request a change in the mandate.
FDA Inspections of Foreign Food Facilities Compared with FSMA Mandate
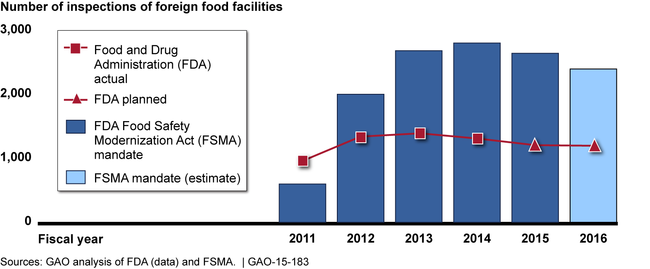
FDA foreign offices cite their contributions to the safety of imported food, but the agency's performance measures do not fully capture these contributions. GAO recommended in 2010 that FDA develop performance measures that can be used to demonstrate the offices' contributions to imported food safety. This recommendation remains valid. FDA has initiated a review to determine how to better reflect the value of the foreign offices in the agency-wide performance systems. Until the offices' contributions are captured, FDA will have less information to effectively measure their progress toward meeting agency goals.
FDA has taken some steps to address recruitment challenges since GAO last reported, but it still does not have a strategic workforce plan. In 2010, GAO recommended that FDA develop such a plan for the foreign offices to help ensure that it recruits and retains staff with the necessary experience and skills. GAO continues to believe that such a plan for the foreign offices is critical to FDA's ability to address staffing challenges, especially since 44 percent of foreign office positions were vacant as of October 2014.
Why GAO Did This Study
FDA has responsibility for ensuring the safety and proper labeling of more than 80 percent of the U.S. food supply, including an increased volume of imported food. Beginning in 2008, FDA established foreign offices to help prevent unsafe products from reaching U.S. borders. In 2010, GAO examined FDA's foreign offices and found that they engaged in a variety of activities relating to food safety but faced challenges due to an increasing workload and other factors. GAO was asked to follow up that report.
This study examines (1) the activities FDA foreign offices have engaged in since 2010 to help ensure the safety of imported food, (2) the extent of the foreign offices' contributions to the safety of imported food, and (3) the extent to which FDA has engaged in workforce planning for its foreign offices. GAO reviewed documentation of foreign office activities and plans, visited offices in China and Mexico, and interviewed agency officials, foreign regulators, and other stakeholders.
Recommendations
GAO recommends that FDA complete an analysis to determine the annual number of foreign food inspections that is sufficient to ensure comparable safety of imported and domestic food. FDA agreed with GAO's recommendation.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Food and Drug Administration |
Priority Rec.
To help ensure the safety of food imported into the United States, the Commissioner of Food and Drugs should complete an analysis to determine the annual number of foreign food inspections that is sufficient to ensure comparable safety of imported and domestic food. If the inspection numbers from that evaluation are different from the inspection targets mandated in FSMA, FDA should report the results to Congress and recommend appropriate legislative changes. |
Open
On March 25, 2020, GAO staff met with FDA officials to discuss the status of the recommendation. FDA officials said that they cannot meet the number of foreign inspections required under the FDA Food Safety Modernization Act (FSMA) due to capacity constraints, and FDA's current strategy for the safety of imported food relies on a "cumulative oversight" approach involving multiple programs, in addition to foreign inspections. FDA officials said that it could be a number of years before these programs are fully implemented and that FDA will provide GAO with more specific status updates on the implementation and monitoring of each of these programs in future responses to this recommendation. In August 2020, FDA officials told GAO that FDA is developing a model to assess its cumulative oversight approach related to allocating oversight resources to ensure that imported and domestic food meet FDA food safety requirements. In FY22, FDA completed 313 foreign food facilities inspections. As of January 2023, FDA had completed 243 foreign food facility inspections. In FY22, FDA completed 95 foreign Remote Regulatory Assessments (RRAs) and, as of January 2023, FDA had completed three foreign RRAs. As of July 2023, FDA had prepared an analysis of all foreign shipments of imported food combined with an analysis of the official establishment inventory and food facility registration information. The FDA has completed an analysis to determine a range of foreign food inspections needed to ensure comparable safety of imported and domestic food. However, FDA has not provided information on how they evaluate the oversight of active foreign food suppliers identified in the analysis to ensure they are performing as expected. Until this information is provided and FDA reports to Congress its assessment regarding the appropriate number of foreign inspections necessary to ensure comparable safety of imported and domestic food, this recommendation will remain open. As of February 2024, FDA has not determined the annual number of foreign food inspections that is sufficient to ensure comparable safety of imported and domestic food, if the inspection numbers from that evaluation are different from the inspection targets mandated in FSMA, nor reported this information to Congress, recommending any identified legislative changes.
|
