Prescription Opioids: Medicare Needs to Expand Oversight Efforts to Reduce the Risk of Harm
Fast Facts
More than 14 million people received opioid prescriptions in 2016 through the Medicare drug benefit program, Part D.
The Centers for Medicare & Medicaid Services delegate monitoring of these beneficiaries to the private organizations that implement the Medicare Part D benefit. These organizations follow CMS monitoring criteria.
While these criteria identify beneficiaries at the greatest risk of harm from opioid use, we found that it does not capture data on an even larger population of beneficiaries potentially at risk of harm from high doses of opioids. We recommended three actions to improve oversight.
Photo of opioid pills
On November 19, 2018, GAO posted an HTML version of this report.
Highlights
What GAO Found
The Centers for Medicare & Medicaid Services (CMS) provides guidance on the monitoring of Medicare beneficiaries who receive opioid prescriptions to plan sponsors—private organizations that implement the Medicare drug benefit, Part D—but lacks information on most beneficiaries at risk of harm.
- CMS provides plan sponsors guidance on how they should monitor opioid overutilization among Medicare Part D beneficiaries and requires them to implement drug utilization review systems that use criteria similar to CMS's. CMS's criteria focus on beneficiaries who (1) receive prescriptions of high doses of opioids, (2) receive prescriptions from four or more providers, and (3) fill the prescriptions at four or more pharmacies. According to CMS officials, this approach allows plan sponsors to focus their actions on those beneficiaries it determined to have the highest risk of harm from opioid use.
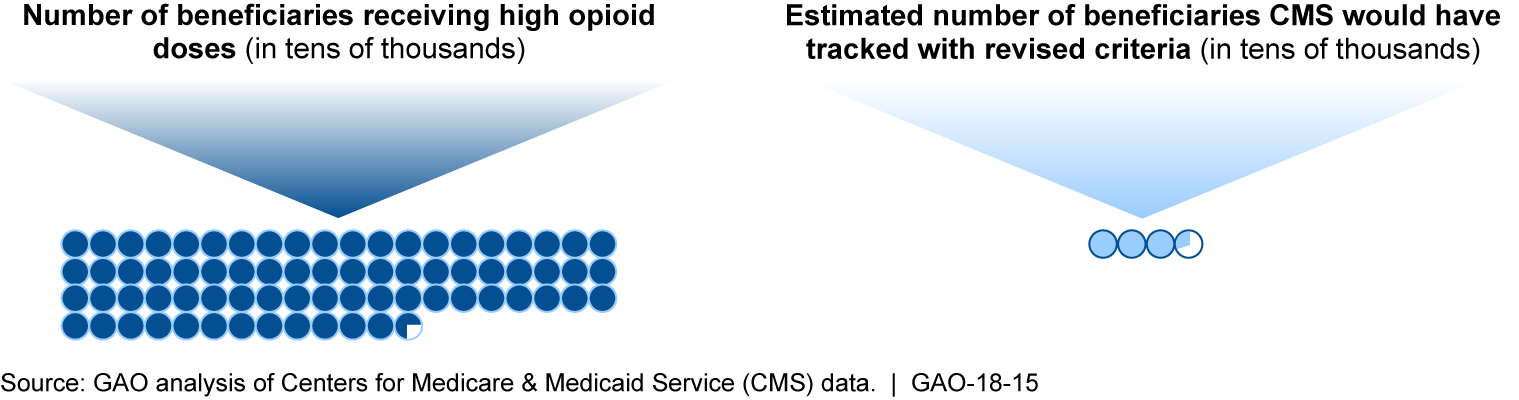
- CMS’s criteria, including recent revisions, do not provide sufficient information about the larger population of potentially at-risk beneficiaries. CMS estimates that while 33,223 beneficiaries would have met the revised criteria in 2015, 727,016 would have received high doses of opioids regardless of the number of providers or pharmacies. In 2016, CMS began to collect information on some of these beneficiaries using a higher dosage threshold for opioid use. This approach misses some who could be at risk of harm, based on Centers for Disease Control and Prevention guidelines. As a result, CMS is limited in its ability to assess progress toward meeting the broader goals of its Opioid Misuse Strategy, which includes activities to reduce the risk of harm from opioid use.
CMS Estimates of 2015 Part D Beneficiaries with High Opioid Doses and Those Who Would Have Met Revised Overutilization Monitoring Criteria
CMS oversees the prescribing of drugs at high risk of abuse through a variety of projects, but does not analyze data specifically on opioids. According to CMS officials, CMS and plan sponsors identify providers who prescribe large amounts of drugs with a high risk of abuse, and those suspected of fraud or abuse may be referred to law enforcement. However, GAO found that CMS does not identify providers who may be inappropriately prescribing large amounts of opioids separately from other drugs, and does not require plan sponsors to report actions they take when they identify such providers. As a result, CMS is lacking information that it could use to assess how opioid prescribing patterns are changing over time, and whether its efforts to reduce harm are effective.
Why GAO Did This Study
Misuse of prescription opioids can lead to overdose and death. In 2016, over 14 million Medicare Part D beneficiaries received opioid prescriptions, and spending for opioids was almost $4.1 billion. GAO and others have reported on inappropriate activities and risks associated with these prescriptions, such as receiving multiple opioid prescriptions from different providers.
GAO was asked to describe what is known about CMS’s oversight of Medicare Part D opioid use and prescribing. This report examines (1) CMS oversight of beneficiaries who receive opioid prescriptions under Part D, and (2) CMS oversight of providers who prescribe opioids to Medicare Part D beneficiaries.GAO reviewed CMS opioid utilization and prescriber data, CMS guidance for plan sponsors, and CMS’s strategy to prevent opioid misuse. GAO also interviewed CMS officials, the six largest Part D plan sponsors, and 12 national associations selected to represent insurance plans, pharmacy benefit managers, physicians, patients, and regulatory and law enforcement authorities.
Recommendations
GAO recommends that CMS (1) gather information on the full number of at-risk beneficiaries receiving high doses of opioids, (2) identify providers who prescribe high amounts of opioids, and (3) require plan sponsors to report to CMS on actions related to providers who inappropriately prescribe opioids. HHS concurred with the first two recommendations, but not with the third. GAO continues to believe the recommendation is valid, as discussed in the report.
Recommendations for Executive Action
| Agency Affected | Recommendation | Status |
|---|---|---|
| Centers for Medicare & Medicaid Services |
Priority Rec.
The Administrator of CMS should gather information over time on the number of beneficiaries at risk of harm from opioids, including those who receive high opioid morphine equivalent doses regardless of the number of pharmacies or providers, as part of assessing progress over time in reaching the agency's goals related to reducing opioid use. (Recommendation 1) |
HHS concurred with this recommendation. Beginning in April 2019, CMS has started using revised measures to track high-risk beneficiaries who use 90 MME per day or more of opioids, regardless of the number of providers or pharmacies used to obtain the medications.
|
| Centers for Medicare & Medicaid Services | The Administrator of CMS should require its contractor, National Benefit Integrity Medicare Drug Integrity Contractor, to identify and conduct analyses on providers who prescribe high amounts of opioids separately from providers who prescribe high amounts of any Schedule II drug. (Recommendation 2) |
HHS concurred with this recommendation. In May and July 2019, HHS provided documentation that CMS, through its contractor, National Benefit Integrity Medicare Drug Integrity Contractor, has , over several quarters in 2018 and 2019, analyzed prescribing data to create a list, or outlier report, of those providers who prescribe high amounts of opioids separately from those who prescribe high amounts of any Schedule II drug.
|
| Centers for Medicare & Medicaid Services | The Administrator of CMS should require plan sponsors to report to CMS on investigations and other actions taken related to providers who prescribe high amounts of opioids. (Recommendation 3) |
Beginning on January 1, 2022, plan sponsors are required to report actions related to inappropriate opioid prescribing to CMS via a Fraud, Waste, and Abuse Portal.
|
